The lab report below was submitted as part of the coursework for
CM2111 Inorganic Chemistry. Please do not plagiarise from it as
plagiarism might land you into trouble with your university. Do note
that my report is well-circulated online and many of my juniors have
received soft copies of it. Hence, please exercise prudence while
referring to it and, if necessary, cite this webpage.
Aim
To examine the effects of the axial ligands on the electronic structures of 4 prepared Ni(II) tetragonal complexes, with electronic spectroscopy and magnetic susceptibility determination.
Procedures
Preparation of Ni complexes
The samples were prepared carefully, according to instructions from the lab manual.
Investigations of the complexes – Electronic Spectra
0.1194g of sample A synthesised, Ni(Et2en)2Cl2.2H2O, was weighed using the 10ml dry volumetric flask before methanol was added with a dropper, until the volume made up to the mark of the volumetric flask. The procedures were repeated, this time with 0.1222g of sample B synthesised, Ni(Et2en)2(NCS)2, and CH2Cl2 as solvent. Both of the volumetric flasks were shaken before pouring each into a clean cuvette to record their electronic spectra.
Investigations of the complexes – Magnetic Properties
All four complexes were first grinded to a fine, uniform powder. Thereafter, the mass of the clean, dried sample tube was first determined. Each sample was then loaded into the tube, with gentle tapping before the next addition to ensure close and even packing. Next, the mass of the sample tube with the sample was weighed to get the mass of each sample present in the tube as well as measuring the length till which the sample filled the sample tube. The magnetic moments of the complexes were then determined using the magnetic susceptibility balance.
Results and calculations
- Ni(Et2en)2Cl2.2H2O
Mass of NiCl2.6H2O used /g
|
0.79
|
Mass of Et2en used /g
|
0.77
|
Mass of Ni(Et2en)2Cl2.2H2O formed / g
|
1.24
|
NiCl2.6H2O + 2Et2en Ni(Et2en)2Cl2.2H2O + 4H2O
Mr. of NiCl2.6H2O = 58.69 + 2 × 35.45 + 6 × [2 × (1.01) +16.00]= 237.71
No. of moles of NiCl2.6H2O used = mass / Mr = 0.79 / 237.71 = 3.32 x 10-3 mol
Mr of Et2en, (CH3CH2)2NCH2CH2NH2 = 14.01 x 2 + 12.01 x 6 + 16 x 1.01= 116.24
No. of moles of Et2en used = mass / Mr = 0.77 / 116.24 = 6.62 x 10-3 mol
No. of moles of NiCl2.6H2O required = ½ x 6.62 x 10-3 = 3.31 x 10-3 mol Et2en is the limiting reagent.
No. of moles of Ni(Et2en)2Cl2.2H2O formed = 3.31 x 10-3 mol
Mr of Ni(Et2en)2Cl2.2H2O = 58.69 +116.24 x 2 + 35.45 x 2 +2 x (16.00+2 x 1.01) = 398.11
Theoretical yield of Ni(Et2en)2Cl2.2H2O = 3.31 x 10-3 x 398.11 = 1.32 g
Percentage yield of Ni(Et2en)2Cl2.2H2O =1.24 / 1.32 x 100 % = 93.9%
- Ni(Et2en)2(NCS)2
Mass of NaNCS used /g
|
0.54
|
Mass of Et2en used /g
|
0.77
|
Mass of Ni(Et2en)2(NCS)2 formed /g
|
1.27
|
Ni(NO3)2.6H2O + 2NaNCSà Ni(NCS)2.4H2O + 2H2O + 2NaNO3
Mr of Ni(NO3)2.6H2O = 58.69 + 2 × 14.01 + 6 × 16.00 + 6 x [2 × (1.01) +16.00]= 290.83
No. of moles of Ni(NO3)2.6H2O used = mass / Mr = 0.97 / 290.83 = 3.34 x 10-3 mol
No. of moles of NaNCS used = mass / Mr = 0.54 / (22.99 + 14.01 + 12.01 + 32.07) = 6.66 x 10-3 mol
No. of moles of Ni(NO3)2.6H2O required = ½ x 6.66 x 10-3 = 3.33 x 10-3 mol\NaNCS is the limiting reagent.
No. of moles of Ni(NCS)2.4H2O formed = 3.33 x 10-3 mol
Ni(NCS)2.4H2O + 2Et2enà Ni(Et2en)2(NCS)2 + 4H2O
No. of moles of Et2en used = mass / Mr = 0.77 / 116.24 = 6.62 x 10-3 mol
No. of moles of Ni(NCS)2.4H2O required = ½ x 6.62 x 10-3 = 3.31 x 10-3 mol \ Et2en is the limiting reagent.
No. of moles of Ni(Et2en)2(NCS)2 formed = 3.31 x 10-3 mol
Mr of Ni(Et2en)2(NCS)2 = 58.69 + 116.24 x 2+ 2 x (14.01 + 12.01 + 32.07) = 407.35
Theoretical yield of Ni(Et2en)2(NCS)2 = 3.31 x 10-3 x 407.35 = 1.35 g
Percentage yield of Ni(Et2en)2(NCS)2 = 1.27 / 1.35 x 100 % = 94.1%
(C)Ni(Et2en)2(NO3)2
Mass of Ni(NO3)2.6H2O used /g
|
0.97
|
Mass of Et2en used /g
|
0.77
|
Mass of Ni(Et2en)2(NO3)2 formed /g
|
1.10
|
Ni(NO3)2.6H2O + 2Et2enà Ni(Et2en)2(NO3)2 + 6H2O
No. of moles of Ni(NO3)2.6H2O used = mass / Mr = 0.97 / 290.83 = 3.34 x 10-3 mol
No. of moles of Et2en used = mass / Mr = 0.77 / 116.24 = 6.62 x 10-3 mol
No. of moles of Ni(NO3)2.6H2O required = ½ x 6.62 x 10-3 = 3.31 x 10-3 mol \ Et2en is the limiting reagent
No. of moles of Ni(NO3)2.6H2O formed = 3.31 x 10-3 mol
Mr of Ni(Et2en)2(NO3)2 = 58.69 + 116.24 x 2 + 14.01 x 2 + 6 x 16.00 = 415.69
Theoretical yield of Ni(Et2en)2(NO3)2 = 3.31 x 10-3 x 415.69 = 1.38 g
Percentage yield of Ni(Et2en)2(NO3)2 = 1.10 / 1.38 x 100 % = 79.7%
(D)Ni(Et2en)2I2
Mass of NaI used /g
|
1.05
|
Mass of Et2en used /g
|
0.77
|
Mass of Ni(Et2en)2I2 formed /g
|
1.29
|
Ni(NO3)2.6H2O + 2NaIà NiI2.4H2O + 2H2O + 2NaNO3
No. of moles of Ni(NO3)2.6H2O used = mass / Mr = 0.97 / 290.83 = 3.34 x 10-3 mol
No. of moles of NaI used = mass / Mr = 1.00 / (22.99 + 126.90) = 6.67 x 10-3 mol
No. of moles of NaI required = 2 x 3.34 x 10-3 = 6.68 x 10-3 mol \NaI is the limiting reagent
No. of moles of NiI2.4H2O formed = 3.34 x 10-3 mol
NiI2.4H2O + 2Et2enà Ni(Et2en)2I2 + 4H2O
No. of moles of Et2en used = mass / Mr = 0.77 / 116.24 = 6.62 x 10-3 mol
No. of moles of NiI2.4H2O required = ½ x 6.62 x 10-3 = 3.31 x 10-3 mol \ Et2en is the limiting reagent
No. of moles of Ni(Et2en)2I2 formed = 3.31 x 10-3 mol
Mr of Ni(Et2en)2I2 = 58.69 + 116.24 x 2 +126.90 x2 = 544.97
Theoretical yield of Ni(Et2en)2I2 = 3.31 x 10-3 x 544.97 = 1.80 g
Percentage yield of Ni(Et2en)2I2 =1.29 / 1.80 x 100% = 71.7%
Electronic Spectra
In order to prepare 3.00 x 10-2 M solutions,
no. of moles of Ni(Et2en)2Cl2.2H2O / Ni(Et2en)2(NCS)2 needed = 3.00 x 10-2 x 10.0 /1000 = 3.00 x 10-4 mol
Mass of A, Ni(Et2en)2Cl2.2H2O needed = 3.00 x 10-4 x 398.04 = 0.1194 g Mass of A used: 0.1194 g Results from UV-Vis spectra for A:
Wavelength
|
Absorbance
|
671.00 nm
|
0.2390
|
398.00 nm
|
0.6664
|
Mass of B, Ni(Et2en)2(NCS)2 needed = 3.00 x 10-4 x 407.26 = 0.1222 g Mass of B used: 0.1222 g
Results from UV-Vis spectra for B:
Wavelength
|
Absorbance
|
581.50 nm
|
0.3600
|
368.00 nm
|
0.7500
|
Determination of magnetic moment and number of unpaired electrons
Compound
|
A
|
B
|
C
|
D
|
Mass of sample /g
|
0.09030
|
0.0852
|
0.0881
|
0.0892
|
Length /mm
|
19.0
|
20.0
|
19.0
|
16.0
|
Magnetic susceptibility, cg
|
0.074 x10-4
|
0.066 x 10-4
|
0.000 x 10-4
|
0.000 x 10-4
|
- Ni(Et2en)2Cl2.2H2O
cm = cg x MW + cD + cTIP = cg x MW + CNi2+ + 2CCl- + 2CEt2en + 2CH2O
= (0.074 x 10-4) x 398.038 + [12.8 + (2 x 23.4) + (2 x 46) + (2 x 13)] x 10-6
= 3.123 x 10-3 cm3 mol-1
m =  =
=  = 2.730 J T-1
= 2.730 J T-1
m =  2.7302 = n2 + 2n n = 1.91 2
2.7302 = n2 + 2n n = 1.91 2
No. of unpaired electrons in Ni(Et2en)2Cl2.2H2O = 2
- Ni(Et2en)2(NCS)2
cm = cg x MW + cD + cTIP = cg x MW + CNi2+ + 2CNCS- + 2CEt2en
= (0.066 x 10-4) x 407.33 + [12.8 + (2 x 31.0) + (2 x 46)] x 10-6
= 2.855 x 10-3 cm3 mol-1
m =  =
=  = 2.610 J T-1
= 2.610 J T-1
m =  n2 + 2n – 2.6102 = 0 n= 1.80 » 2
n2 + 2n – 2.6102 = 0 n= 1.80 » 2
\No. of unpaired electrons in Ni(Et2en)2(NCS)2 = 2
- Ni(Et2en)2(NO3)2
cm = cg x MW + cD + cTIP = cg x MW + CNi2+ + 2CNO3- + 2CEt2en
= 0 + [12.8 + (2 x 46) + (2 x 18.9)] x 10-6 = 1.426 x 10-4 cm3 mol-1
m =  =
=  = 0.583 J T-1
= 0.583 J T-1
m =  0.5832 = n2 + 2n n= 0.158 » 0
0.5832 = n2 + 2n n= 0.158 » 0
\No. of unpaired electrons in Ni(Et2en)2(NO3)2 = 0
- Ni(Et2en)2I2
cm = cg x MW + cD + cTIP = cg x MW + CNi2+ + 2CI- + 2CEt2en
= 0.000 + [12.8 + (2 x 50.6) + (2 x 46)] x 10-6 = 2.06 x 10-4 cm3 mol-1
m =  =
= 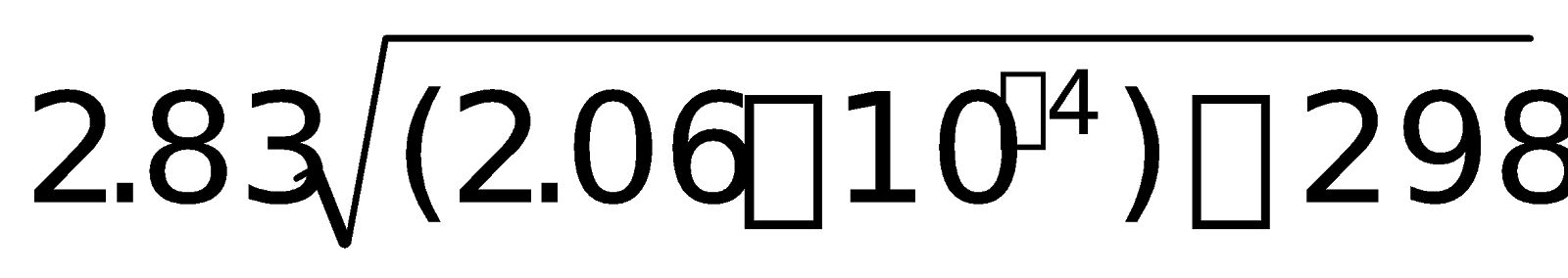 = 0.701 J T-1
= 0.701 J T-1
m =  0.7012 = n2 + 2n n= 0.221 » 0
0.7012 = n2 + 2n n= 0.221 » 0
\No. of unpaired electrons in Ni(Et2en)2I2 = 0
Discussion
Colour of Ni complexes
In this experiment, the obtained Ni(Et2en)2Cl2.2H2O crystals are light-blue; Ni(Et2en)2(NCS)2 are violet crystals; Ni(Et2en)2(NO3)2 is yellow and Ni(Et2en)2I2, red-orange.
These nickel complexes are coloured because they absorb light in the visible region. Absorption of energy from the visible light region of the electromagnetic spectrum occurs when an electron is promoted from a d-orbital in nickel of lower energy to a higher energy. The colour of the compound observed is the complementary colour of the absorbed colour of the visible light.
UV/VIS spectra in terms of electronic structure
Ni(II) has a d8 electronic configuration at ground state with 3F term. The associated energy levels arising from crystal field splitting arranged from low to high are A2g, T2g, T1g(F) and T1g (P). There are only 3 possible transitions arranged from small energy gap to big energy gap: 3T2g 3A2g, 3T1g (F) 3A2g and 3T1g (P) 3A2g. Since the magnitude of wavelength of light absorbed is inversely proportional to the magnitude of the energy gap, hence 3T1g (P) 3A2g occurs at the shortest wavelength followed by 3T1g (F) ß 3A2g then 3T2g ß3A2g.
The spectra of Ni(Et2en)2Cl2.2H2O (as attached) contains 2 peaks at 671.00nm and 398.00nm. The absorbed light of wavelength 671.00nm corresponds to the 3T1g (F) ß 3A2g transition while that at 398.00nm corresponds to the 3T1g (P)ß 3A2g transition.
The spectra of Ni(Et2en)2(NCS)2 (as attached) contains 2 peaks at 581.50nm and 368.00nm. For this nickel(II) complex, the absorbed light of wavelength 581.50nm corresponds to the 3T1g (F) ß 3A2g transition while that at 368.00nm corresponds to the 3T1g (P)ß 3A2g transition.
Ni(II) complexes having d8 configurations with P and F terms can undergo 3 transitions, which correspond to 3 absorption peaks in theory. However, only 2 peaks are present for all the obtained spectra. This is because the wavelength used in the UV-visible spectrophotometer is 350 nm to 800nm and the ‘missing’ peak may lie outside this range.
Et2en is a bidentate ligand that occupies the equatorial position due to the short C-C bond which cannot stretch across axial-equatorial. For these Ni complexes, the main difference among the 4 complexes is the 2 axial ligands. A stronger field ligand will cause a greater splitting of the energy states and hence a greater value for the transitions, translating to more energy required and hence shorter wavelength for absorption to take place. From the spectra, for the same transition of 3T1g (P)ß 3A2g, the maximum absorption occurs at 368.00nm for Ni(Et2en)2(NCS)2, 398.00nm for Ni(Et2en)2Cl2.2H2O, 455.00nm for Ni(Et2en)2(NO3)2 and 475.00nm for Ni(Et2en)2I2 in increasing wavelengths. Hence, it can be deduced that the ligand-field strengths are follows: NCS->Cl->NO3->I-. This observation is in line with the spectrochemical series except for NO3- which is supposed to be stronger than that of Cl-. One possible reason for this deviation could be due to binding competition from the Et2en chelate or affinity of the central Ni ion for certain ligands.
Magnetic properties in terms of electronic configuration
As seen from the calculations above, Ni(Et2en)2Cl2.2H2O and Ni(Et2en)2(NCS)2 are paramagnetic due to the presence of 2 unpaired electrons. It can be inferred that they would most probably adopt the high-spin configuration. However, Ni(Et2en)2(NO3)2 and Ni(Et2en)2I2, with no unpaired electrons, would adopt the low-spin configuration.
Ni(Et2en)2Cl2.2H2O and Ni(Et2en)2(NCS)2 undergo weak tetragonal distortions to form tetragonal geometry while Ni(Et2en)2(NO3)2 and Ni(Et2en)2I2 undergo strong tetragonal distortions to form square planar structures.
For tetragonal complexes, Ni(Et2en)2Cl2.2H2O and Ni(Et2en)2(NCS)2, a1g and b1g orbitals are close in energy, hence the last 2 electrons fill in the orbitals singly rather than pair up in a1g orbitals. There are 2 unpaired electrons in the complexes, therefore accounting for their paramagnetism. This corresponds to experimental findings. The complexes will be attracted to magnetic fields and carry magnetic moment due to the large energy difference resulting from the strong field ligands which causes the pairing energy of the electrons to be negligible and hence the electrons will attain a high spin configuration.
For square planar complexes, Ni(Et2en)2(NO3)2 and Ni(Et2en)2I2 , since b1g and b2g orbitals are far apart in energy, the last 2 electrons will pair up given that the pairing energy is sufficient to compensate for the energy gap to exhibit low spin configuration. There are no unpaired electrons in these 2 complexes, thereby resulting in zero magnetic susceptibility. This corresponds to the experimental results.
As a result, both the electronic configurations of Ni(Et2en)2(NCS)2 and Ni(Et2en)2Cl2.2H2O complexes are eg4 b2g2 a1g1b1g1. The electronic configurations for that of Ni(Et2en)2I2 and Ni(Et2en)2(NO3)2 complexes are both eg4 a1g2b2g2.
Precautions
All apparatus were thoroughly cleaned with deionised water before use and all measurements were taken with minimal human inherent error. Dry and clean volumetric flasks were used for UV spectroscopy since the presence of water could affect the concentrations of the solutions and the spectra obtained. During the transferring of crystals, it was ensured that as little crystals were lost as possible. When using the magnetic susceptibility balance, the sample was dried and powdered before insertion into the tube; it was loaded in small amounts and the tube was tapped gently on a wooden bench to ensure even packing; the sample was at least 15mm in depth. Meticulous packing of the samples into the tube was important to ensure even distribution of magnetic field when operating the magnetic susceptibility balance.
Yields
The yields of the product are in the range of 71.7% to 94.7%; these yields are not 100% because ligand substitution reactions are reversible. Also, some of the products may have also been lost during transferring. To raise the yield, the solution should be allowed to stand for crystallisation longer and a higher concentration of ligands, used. Minimum amount of ethanol should be used for washing as ethanol will dissolve the crystals, thereby reducing the product yield.
Conclusion
The percentage yields of Ni(Et2en)2Cl2.2H2O, Ni(Et2en)2(NCS)2, Ni(Et2en)2(NO3)2 and Ni(Et2en)2I2 are 93.9%, 94.1%, 79.7% and 71.7% respectively. Both Ni(Et2en)2Cl2.2H2O and Ni(Et2en)2(NCS)2 contain 2 unpaired electrons each and exhibit paramagnetism; Ni(Et2en)2(NO3)2 and Ni(Et2en)2I2 ,however, contain no unpaired electron and do not exhibit paramagnetism.
References
- Anonymous, “Crystal Field Theory”, University of West Indies, article retrieved on 25 Jan 2012: http://wwwchem.uwimona.edu.jm/courses/CFT.html
- A. Earnshaw, “Introduction to Magnetochemistry”, Academic Press, 1968
stilimti_pe-1983 Tim Gray https://wakelet.com/wake/PmYqG7x7nNiDLX653_eh6
ReplyDeletetamdroogahve