Lab Report on Preparation and IR Spectral Analysis of Oxovanadium Acetylacetonate and its Pyridine Adduct
The lab report below was submitted as part of the coursework for
CM2111 Inorganic Chemistry. Please do not plagiarise from it as
plagiarism might land you into trouble with your university. Do note
that my report is well-circulated online and many of my juniors have
received soft copies of it. Hence, please exercise prudence while
referring to it and, if necessary, cite this webpage.
Abstract
Oxovanadium complexes exhibit cytotoxic activities against human cancer cell lines. These chemicals are able to kill target cancer cells at low micromolar concentrations[1]. Current literature suggests that the therapeutic characteristics of these metal complexes are influenced by the identity of the five membered bidentate ligands, as well as the nature of the substituents on the heterocyclic ring. [2]
In this experiment, an oxovanadium complex – oxovanadium acetoacetonate-pyridine, VO(acac)2py – was prepared before infrared (IR) spectroscopy was employed to characterise its vibrational energy levels. The spectroscopic results acquired were compared to that provided for VO(acac)2 to determine the magnitude by which the V=O stretching mode has shifted. This analysis allows insights into the chemical properties of an oxovandium complex to be gleaned.
Introduction
Acetylacetone (2,4-pentanedione) is a -diketone. The carbon atom of the CH2 group in between the 2 carbonyl group is α-carbon and the hydrogens attached to it are acidic. This is due to the presence of 2 electron withdrawing carbonyl groups. The α-hydrogen may be easily lost to water to produce an anion stablised by resonance (as shown below):
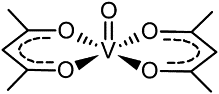
The acetylacetonate anion can then act as a ligand towards the oxovanadium cation to produce VO(acac)2. This ligand bonds to the metal ion through both its oxygen atoms and, hence, a six membered, weakly aromatic ring is produced (as shown below):
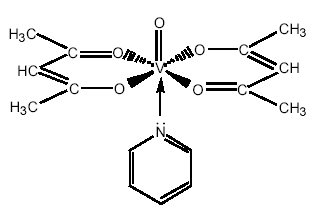
Refluxing with pyridine causes further metal-ligand coordination and produces VO(acac)2py. IR spectroscopy is useful then in distinguishing between the vibrational energies of the dark blue-green VO(acac)2 and greyish VO(acac)2py.
Experimental
Preparation of VO(acac)2
5.0 ml of distilled water was added to a 50 ml round-bottom flask, followed by the careful addition of an equal volume of concentrated H2SO4.
Then, 18.0 ml of ethanol and 2 g of vanadium pentoxide, V2O5, was added to the round-bottom flask. The flask was thereafter connected a water cooled vertical condenser and refluxed vigorously in a heating mantle at a setting of 10 for an hour. The mixture was swirled every 5 minutes. The orange V2O5 solid dissolves in the colourless solvent to produce a dark blue-green solution.
After an hour, the mixture was cooled in water and filtered using vacuum filtration. The solid residue was discarded. The filtrate was poured into a 250ml conical flask. 6 ml of acetylacetone was added dropwise to the filtrate with shaking. The mixture was then neutralised by adding it carefully to a solution of 20 g of hydrated Na2CO3 in 120 ml deionised water, contained in a 500 ml conical flask, while swirling the flask.
The resulting mixture was cooled in an ice water bath, before it was filtered at the pump. The dark green product was washed with cold deionised water and thereafter, sucked dry by the vacuum pump. The dried product was then weighed and the percentage yield, determined.
Preparation of VO(acac)2py
0.5 g of the crystallised blue-green product was dissolved in 10ml toluene in a 50 ml round-bottomed flask. The solution was then refluxed vigorously with 2 ml pyridine for one hour in a heating mantle with setting 10. To concentrate the mixture to a small volume, the round bottom flask was then exposed to rotary evaporation. Subsequently, the round bottom flask was cooled in an ice bath for crystallisation. The crystals were filtered, washed with 5 ml ether and sucked dry. The dry product was weighed and the percentage yield calculated.
Preparation of the sample for recording the infra-red spectrum
Before this phase, it was ensured that the sodium chloride discs, mortar and pestle were wiped clean with clorofoam on tissue. A small amount of the dried compound (about 10 – 20 mg) was then grinded very finely with an agate mortar and pestle. Three drops of Nujol was added with a dropper and the mixture was grinded again. Thereafter, the Nujol mull was carefully spread on the surface of the disc and the discs were inserted into its holder. The infra-red spectrum was obtained and printed.
Results and calculation
Empirical observations
V2O5: orange solid
VO(acac)2: dark blue-green solid
VO(acac)2py: greenish grey solid
Preparation of VO(acac)2
Weight of V2O5 used: 2.00 g
Weight of hydrated Na2CO3: 7.40 g
Weight of dried VO (acac)2: 4.41 g
V2O5 + 4H+ 2(VO)2+ + 2H2O + 1/2O2 (1)
(VO)2+ + 2 acacH 2H+ + VO (acac)2 (2)
No. of moles of V2O5 used = 2.00 / (50.94 x 2 + 5 x 16.00) = 0.01099 mol
Using equation 1 and 2, each mole of V2O5 yields 2 moles of (VO)2+ and hence 2 moles of VO (acac)2.
No. of mole of VO (acac)2 = 0.010996 mol × 2 = 0.02199 mol
Mr of VO(acac)2 = (50.94 + 16.00) + 2×(5 × 12.01 + 7 × 1.008 + 2 × 16.00) = 265.15 g mol-1
Theoretical yield of VO(acac)2 = (265.15 g/mol) × 0.021992 mol = 5.831 g
Percentage yield of VO (acac)2 = 4.41 / 5.831 x 100% ≈ 75.6 %
Preparation of VO(acac)2py
Mass of VO(acac)2 product used
|
0.50 g
|
Mass of dry product, VO(acac)2py
|
0.41 g
|
Mr of VO(acac)2py = 265.16 + 5 × 12.01 + 5 × 1.008 + 14.01 = 344.26 g mol-1
VO(acac)2 + py VO(acac)2py
No. of moles of VO(acac)2 used = No.of moles of VO(acac)2py = (0.50/265.15)= 0.001886 mol
Theoretical yield of VO(acac)2py = 344.26 × 0.001886 = 0.6492 g
Percentage yield of VO(acac)2py = 0.41/0.6492 × 100% = 63.2%
Infrared Spectra
By comparison of spectrum obtained with IR spectrum of VO(acac)2 provided,
Spectrum obtained, VO(acac)2py
|
Sample spectrum, VO (acac)2
| |
V=O stretching
|
965.3 cm-1
|
997 cm-1
|
Amount by which the band shifted = 997 – 965.3 = 31.7 cm-1
Discussion
Formation of VO (acac)2 and VO(acac)2py
Refluxing V2O5 with concentrated H2SO4, water and ethanol for an hour, reduces V2O5 to (VO)2+, as described in the equation below. Orange V2O5 dissolves in the colourless solvents to form a dark green (VO)2+ solution.
V2O5 + 4H+ -> 2(VO)2+ + 2H2O + 1/2O2
The vanadium (II) ion was chelated with 2 acac– anions to form blue-green [VO(acac)2]. This complex has a square-based pyramidal geometry and is able to accommodate one more ligand.
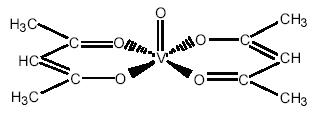
(VO)2+ + 2 acacH -> 2H+ + VO (acac)2
Na2CO3 was added in order to remove the excess H+ from the ionisation of acacH and concentrated H2SO4 so as to drive the reaction to completion. By Le Chateliers principle, with the removal of H+ ions, the equilibrium of the reaction is shifted to favour the formation of the VO(acac)2 complex.
Pyridine has a lone pair of electrons on the N atom which can be used to bind with the central vanadium ion to form a 6-coordinated complex. The overall structure of the grey complex, VO(acac)2py, will be octahedral (image, right).
Colours of products
V2O5, VO (acac)2 and VO(acac)2py are coloured because they absorb light in the visible region. The energy of the absorbed photon corresponds to the energy gap between vanadium ion’s d-orbitals. The colour of the compound observed is complementary to that of the absorbed light.
Percentage yield of products
The percentage yield of the product, VO(acac)2, was a reasonable 75.6%. However, it was not 100%. This may be due to improper mixing of the reagents within the round bottom flask which resulted in lesser amount of V2O5 reacting to produce lesser VO2+ – which is subsequently required to form VO(acac)2. The yield could be increased by refluxing the mixture longer and ensuring more thorough swirling to allow more conversion of V2O5 to VO(acac)2. More time is required to leave the mixture to stand in an ice bath for crystallisation after Na2CO3 is added in order to produce a higher yield of VO(acac)2. More base may be added to favour the formation of the desired product as well.
The percentage yield of VO(acac)2py was slightly lower at 63.2%. This may be attributed to the unfavourable steric effect of attaching the bulky pyridine ligand to the oxovanadium ion with 2 acac chelates. Also, metal-ligand coordination is reversible[3] and hence, the reaction only proceeded partially to form the octahedral oxovanadium complex. A rotary evaporator was used to remove excess solvents by making use of vacuum without excessive heating as excessive heating may decompose the product. Also, some products adhered to the round bottom flask after rotary evaporation, which contributed to the mediocre yield.
Loss of product during weighing, suction filtration and transferring from one container to another have also occured.
Infrared spectroscopy
From the IR spectra, it was observed that the V=O stretching mode of VO(acac)2 was at 997cm-1 while that of VO(acac)2py, 965.3cm-1. The addition of pyridine into the square geometric VO(acac)2 complex caused the vibration frequency of V=O stretching to decrease by 31.7 cm-1. When the pyridine forms coordinate bond with the VO(acac)2, it introduces a lone pair of electrons from nitrogen into the d-orbital of the oxovanadium ion. This increases the electron density around the vanadium ion and thus, results in electron-electron repulsion between the V=O bond. To minimise the repulsion, V=O bond lengthens and weakens. Therefore, the band shifts to a lower wavenumber.
Significant peaks in VO(acac)2 (cm-1)
|
Significant peaks in VO(acac)2py (cm-1)
|
Comments
|
2924, 2954
|
2925.0, 2854.2
|
Peaks remained as they are due to Nujol
|
1559, 1531
|
-
|
Peaks disappeared.
|
1462
|
1462.2
|
Peaks remained.
|
1376
|
1377.2
|
Peaks remained as they are due to Nujol.
|
1021
|
1023.4
|
Peaks remained.
|
997
|
965.3
|
Peaks shifted by 31.7 cm-1.
|
There are not many significant differences between the 2 spectra of the VO(acac)2 complex and the pyridine adduct except the disappearance of some peaks at 1569 cm-1 and 997 cm-1. This may be attributed to the presence of pyridine which affects the absorption peaks due to the distortion of the adjacent bonds. The disappearance of the 997cm-1 peak shows that there is complete reaction of VO(acac)2. In general, the two spectra are fairly similar for the 2 complexes have similar structures.
Precautions
As the reagents may be toxic, gloves were worn to protect the hands. This is especially important when handling corrosive reagents such as concentrated sulphuric acid. The experiment was carried out in the fumehood to prevent any inhalation of harmful organic vapours. The flammable reagents (pyridine, toluene and acetylacetone) were also placed away from naked flames.
Experimental techniques
To encourage even boiling, pieces of boiling chips were added to the round bottom flask during reflux.
Also, the solution of (VO)2+ was not filtered with cotton wool, which was suggested by the lab manual. Instead, filter papers were used. This was because cotton cool may be easily dislodged and thus, allow unreacted V2O5 to pass through. By contrast, filter paper is more effective at separating the insoluble unreacted reagent and soluble product[4] .
A magnetic stirrer should be added to the round bottom flask to further ensure homogenous mixing of the reagents, thereby speeding up the reaction.
Conclusion
The preparation and chemical analysis of oxovanadium acetylacetonate and its pyridine adduct allow their chemical properties to be characterised. The percentage yield of VO(acac)2 was 75.6% while that of VO(acac)2py, 63.2%. The V=O stretching shifted from 997 cm-1 in VO (acac)2 to 965.3 cm-1 in VO(acac)2py.
References
[1] Bis(4,7-dimethyl-1,10-phenanthroline) Sulfatooxovanadium(IV) as a Novel Apoptosis-inducing Anticancer Agent. D’Cruz J. Osmond, Dong Yanhong, Rama Krishna Narla and Uckun Fatih M. American Association for Cancer Research, 2007, article retrieved on 12/02/12:
[2] Antitumor activities of vanadium(IV), manganese(IV), iron(III), cobalt(II) and copper(II) complexes of 2-methylaminopyridine. El-Naggar M. M., El-Waseef A. M., El-Halafawy K. M., El-Sayed I. H. Cancer Lett., 133: 71-76, 1998.
[3] Coordination Chemistry: Reactions of Complexes. Shriver and Atkins. Inorganic Chemistry, 5th Edition, Oxford University Press, 2009.
[4] The Interactive Chemistry Laboratory Manual (ICLM), reference retrieved on 14 Jan 2012: http://courseware.nus.edu.sg/iclm/default.asp
This report should NOT be used for report writing. I would reccomment using an Inorganic textbook like Shriver and Atkins. I mark reports and am aware of this page. I report any students using it for plagerism. This may have been posted with good intensions but it isn't even a very good report. It is much better to do your own work and learn from it. First year labs are not worth so much it is worth cheating on.
ReplyDeleteYou're right :]
DeleteStudents should definitely do their own work; that's how they learn. I certainly hope that they won't plagiarise!
This lab report is very good guide for a first year student. I repeat as a GUIDE! I too mark lab reports and using this is not bad as long as you reference if used in your work. The Shriver and Atkins book is also good but this is a good indication of how to set a lab report.
ReplyDeleteThanks!
DeleteEver since I graduated, I don't have much use for these lab reports. Hope it helps busy undergraduates by offering a starting point :]
Very efficiently written information. It will be beneficial to anybody who utilizes it, including me. Keep up the good work. For sure i will check out more posts. This site seems to get a good amount of visitors. 4 aco dmt kaufen
ReplyDeleteThe blog was having very informative content and very useful for me. Well done post and keep it up... Thanks for sharing such a Useful info.dmt herstellung
ReplyDelete