The lab report below was submitted as part of the coursework for
CM3291 Advanced Experiments in Organic and Inorganic Chemistry. Please do not plagiarise from it as
plagiarism might land you into trouble with your university. Do note
that my report is well-circulated online and many of my juniors have
received soft copies of it. Hence, please exercise prudence while
referring to it and, if necessary, cite this webpage.






Aim
To carry out a multistep synthesis of p-Tolilic acid from p-Tolualdehyde. The first step involves the preparation of 4,4’-dimethylbenzoin from p-Tolualdehyde using thiamine hydrochloride as the catalyst. In the subsequent step, the 4,4-dimethylbenzoin is converted to 4,4’-dimethylbenzil using copper(II) acetate as the catalyst. Finally, the 4,4’-dimethylbenzil is used to prepare p-Tolilic acid by reaction in basic conditions followed by an acidic work-up. The IR and melting point of the p-Tolilic acid was also determined.
Results & Calculations
Part A: Preparation of 4,4’-dimethlbenzoin from p-Tolualdehyde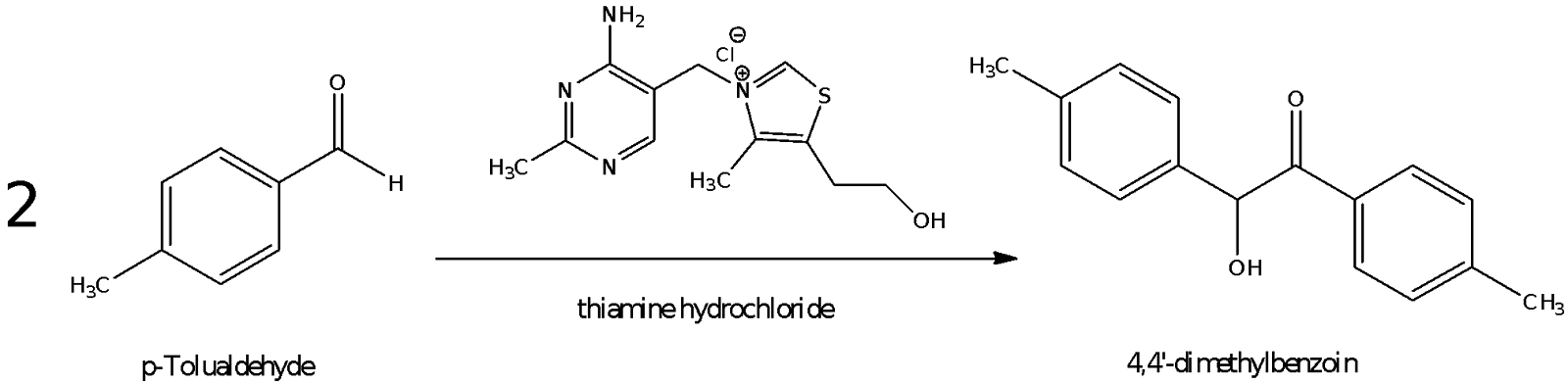
Mass of thiamine hydrochloride: 1.9858 g
Volume of p-Tolualdehyde: 7.01 ml
Density of p-Tolualdehyde: 1.015 g ml-1
Mass of p-Tolualdehyde: 1.015 x 7.00 = 7.1152 g
Molar mass of p-Tolualdehyde: 8 x 12.01 + 8 x 1.0079 + 16.00 = 120.14 g mol-1
No. of moles of p-Tolualdehyde: 7.1152 / 120.14 = 0.05922 mol
Mole ratio of p-Tolualdehyde to 4,4’-dimethylbenzoin is 2:1.
Expected no. of moles of 4,4’-dimethylbenzoin: 0.05922 / 2 = 0.02961 mol
Molar mass of 4,4’-dimethylbenzoin: 16 x 12.01 + 16 x 1.0079 + 2 x 16.00 = 240.29 g mol-1
Expected mass of 4,4’-dimethylbenzoin produced: 0.02961 x 240.29 = 7.1155 g
Mass of 4,4’-dimethylbenzoin obtained: 4.7308 g
Percentage yield of 4,4’-dimethylbenzoin: 4.7308 / 7.1155 x 100% = 66.5%
Part B: Preparation of 4,4’-dimethylbenzil from 4,4’-dimethylbenzoin
Mass of copper (II) acetate: 0.0603 g
Mass of 4,4’-dimethylbenzoin used: 4.7305 g
No. of moles of 4,4’-dimethylbenzoin: 4.7305 / 240.29 = 19.686 mmol
Mass of ammonium nitrate: 2.3652 g
Mole ratio of 4,4’-dimethylbenzoin to 4,4’-dimethylbenzil is 1:1.
Expected no. of moles of 4,4’dimethylbenzil: 19.686 mmol
Molar mass of 4,4’-dimethylbenzil: 16 x 12.01 + 14 x 1.0079 + 2 x 16.00 = 238.27 g mol-1
Expected mass of 4,4’-dimethylbenzil: 19.686 x 10-3 x 238.27 = 4.6907 g
Mass of 4,4’-dimethylbenzil obtained: 2.901 g
Percentage yield of 4,4’-dimethylbenzil: 2.901 / 4.6907 x 100% = 61.8%
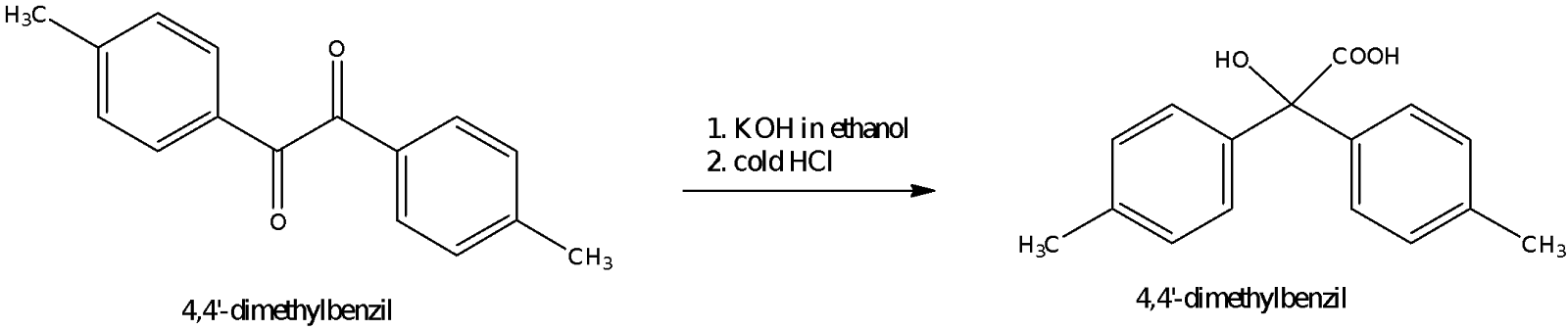
Part C: Preparation of p-Tolilic acid from 4,4’-dimethylbenzil
Mass of 4,4’-dimethylbenzil used: 2.850 g
No. of moles of 4,4’-dimethylbenzil: 2.850 / 238.27 = 11.961 mmol
Mole ratio of 4,4’-dimethylbenzil to p-Tolilic acid is 1:1.
Molar mass of p-Tolilic acid: 16 x 12.01 + 16 x 1.0079 + 3 x 16.00 = 256.29 g mol-1
Expected mass of p-Tolilic acid: 11.961 x 10-3 x 256.29 = 3.0655 g
Mass of p-Tolilic acid obtained 0.1120 g
Percentage yield of p-Tolilic acid: 0.1120 / 3.0655 x 100% = 3.65%
Melting point of product: 126.80 – 127.50 oC
Overall yield
Expected mass of p-Tolilic acid: 0.05922/2 x 256.29 = 7.5887 g
Overall yield = 0.1120 / 7.5887 x 100% = 1.48%
Discussion
In the first part of the experiment, 4,4’-dimethylbenzoin was prepared from p-Tolualdehyde using thiamine hydrochloride as the catalyst. Thiamine hydrochloride was first dissolved in water to yield chloride ions and thiamine. Upon addition of ethanol, a white precipitate forms because thiamine is less soluble in ethanol. Next, NaOH solution was added and this creates a basic environment changing the structure of a thiamine. The white precipitate dissolves and form a yellow solution. Thiamine reacts with hydroxyl ions in basic solutions in the following manner:
Figure 1: Thiamine under basic conditions
Under basic conditions, the amino group in thiamine attacks the carbon-nitrogen double bond in a nucleophilic reaction, causing the loss of a proton and yielding a tricyclic dihydrothiachromine intermediate form. As it is an intramolecular nucleophilic attack, the reaction occurs rapidly and in preference to an external attack by an OH- at the same electrophilic carbon. Subsequently, another proton is lost in the second step to open the thiazole ring to form the yellow thiol form. The presence of this thiol gives rise to the yellow colour of the solution observed.
For the reaction to form 4,4’-dimethylbenzoin, an ylid is first formed from thiamine. An acidic proton is extracted by the hydroxyl ions present in the solution to form the ylid.
Figure 2: Formation of the ylid
The ylid is stabilized by the adjacent electronegative atoms of N and S. The positive charge on nitrogen further draws away the electron density from the carbon which further stabilizes the ylid. To this ylid that is present in solution, p-tolualdehyde was added to it.
Figure 3: Mechanism for Part A reaction (benzoin condensation)
The mechanism for the reaction is that of a thiamine-catalyzed benzoin condensation reaction. The nucleophilic ylid formed by deprotonation of thiamine attacks the p-tolualdehyde at the electrophilic carbonyl carbon to form the tetrahedral intermediate. From this intermediate, the oxoanion extracts the adjacent acidic proton whilst forming an enamine in the process. The enamine then acts as a nucleophile to add on another equivalent of p-tolualdehyde by similarly attacking the electrophilic carbonyl carbon. Eventually, the lone pair on the hydroxyl group donates itself to a bond and kicks out the ylid to give the products of 4,4’-dimethylbenzoin and the ylid catalyst. Overall, two equivalents of p-tolualdehyde have been condensed to give one equivalent of 4,4’-dimethylbenzoin.
Thiamine, with the common name of vitamin B1, decomposes readily upon heating hence the reaction mixture was cooled in an ice/water bath to prevent this. NaOH was added until the pH was equal to or above 9 to ensure that the thiamine could be deprotonated by the base to form the ylid. The composition of the reaction mixture also needed to be taken into account because an overly aqueous medium would prevent the p-tolualdehyde from being present in the solution. The p-tolualdehyde is slightly soluble in aqueous medium which was why only 3 ml of water and about 5 ml of NaOH was added. 20 ml ethanol was used to form the bulk of the mixture and to enable p-tolualdehyde to dissolve in it more readily as ethanol is a polar solvent. Furthermore, ethanol is miscible with water and the thiamine present in water can interact freely with the p-tolualdehyde in ethanol. The reaction mixture was stored in the dark to prevent p-tolualdehyde from being oxidised to p-toluic acid by exposure to light. The reaction took place at room temperature hence the rate of reaction was slow, and it was left to react for a week to ensure the reaction goes to completion as much as possible.
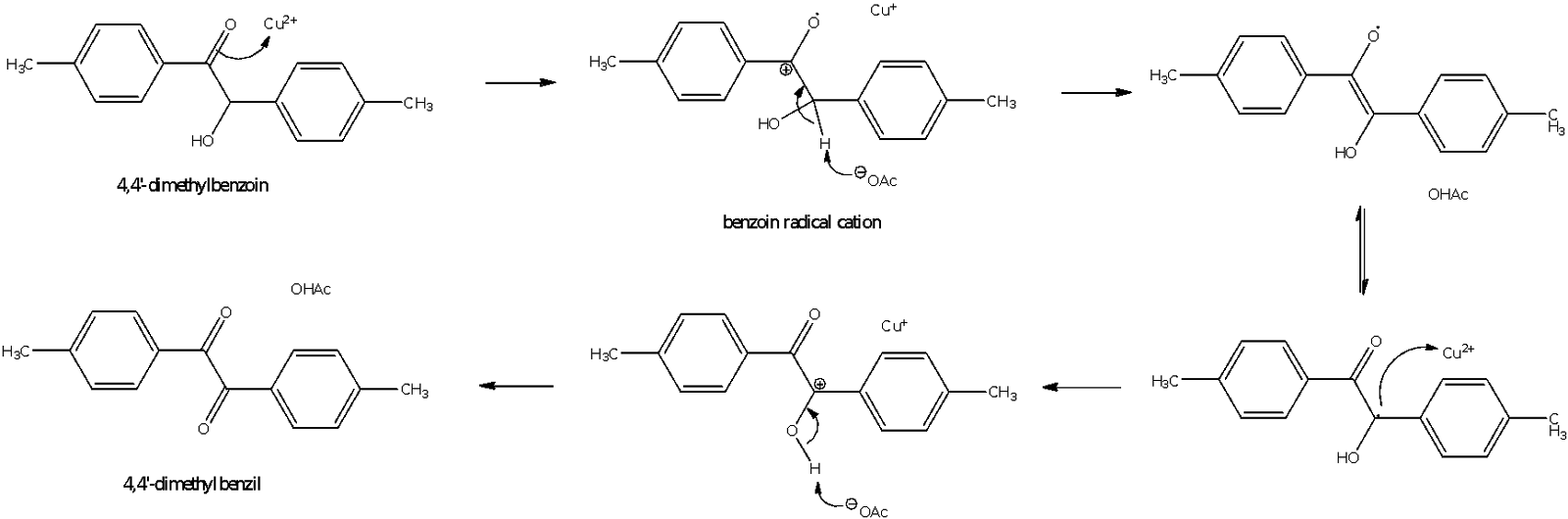
In part B of the experiment, 4,4’-dimethylbenzoin that was obtained from part A was oxidized to 4,4’-dimethylbenzil using a Cu2+ salt and ammonium nitrate. The Cu2+ salt here is Cu(OAc)2 and only catalytic amounts of Cu2+ are necessary because they are continuously recycled. The Cu+ ions formed by reaction with 4,4’-dimethylbenzoin are reoxidized to Cu2+ by ammonium nitrate which is present in excess. The overall reaction can be summed up as two paired redox reactions taking place as shown by the diagram on the left. A stronger oxidising agent like potassium dichromate was not used as it might cause the cleavage of the C-C bond in the benzoin molecule, causing the reformation of p-tolualdehyde instead. Meanwhile, the entire reaction mechanism is depicted as follows:
Figure 4: Mechanism for Part B reaction (oxidation of 4,4’-dimethylbenzoin to 4,4’-dimethylbenzil)
In the first redox cycle, the 4,4’-dimethylbenzoin donates an electron to Cu2+, forming Cu+ and a benzoin radical cation. The benzoin radical cation then loses a proton to acetate ion to form acetic acid and a resonance-stabilized radical. Another redox cycle between Cu2+ and the radical takes place, forming a second Cu+ ion and cation. This cation then loses a proton to another acetate ion, forming 4,4’-dimethylbenzil. Meanwhile, for the second redox cycle, the Cu+ is reoxidized back to Cu2+ by NH4NO3 which acts as an oxidizing agent:
Figure 5: Second redox cycle – Cu+ to Cu2+
The mixture was refluxed for an hour to speed up the oxidation reaction. It could be observed that for every equivalent of 4,4’-dimethylbenzoin that was oxidized to 4,4’-dimethylbenzil, 2 equivalents of Cu2+ were required and this was regenerated by 1 equivalent of NH4NO3. Hence, the NH4NO3 added was roughly 0.5 by mass of the benzoin added, which is more than the required molar equivalent of NH4NO3 required. After reflux, the reaction mixture was poured unto crushed ice to precipitate out the 4,4’-dimethylbenzil which is insoluble in water. 
Figure 6: Mechanism for Part C reaction (oxidation of 4,4’-dimethylbenzil to p-Tolilic acid)
For part C of the experiment, p-Tolilic acid was prepared from 4,4’-dimethylbenzil using an potassium hydroxide solution which was mixed with ethanol. The reaction is known as a benzilic acid rearrangement because a phenyl group migrates from one carbon to another. The mechanism for the reaction is as shown in Figure 6.
The first step involves the attack by the nucleophile OH- on one of the electrophilic carbonyl carbons in a nucleophilic addition reaction to form a tetrahedral intermediate. The next step involves a conformational change or a rearrangement as the phenyl group migrates to the other carbonyl carbon as the tetrahedral intermediate collapses. The reaction is analogous of a semipinacol rearrangement in which a deprotonated hydroxyl group provides the impetus to push the phenyl group to the other carbon and at the same time, the electrons in the carbonyl bond migrate to the carbonyl oxygen atom to make way for the incoming group. A semipinacol rearrangement involves a carbocation adjacent to a carbon with a hydroxyl group. The rearrangement involves the transfer of an R group from the carbon with the hydroxyl group to the carbocation. As such, the semipinacol rearrangement is similar to that of the benzilic rearrangement reaction. After the rearrangement, a proton transfer occurs in which the more basic oxoanion pulls the proton from the carboxylic acid in an intramolecular deprotonation. The proton could also have come from a molecule of water instead of from the carboxylic acid group. This deprotonated p-Tolilic acid was then acidified by treating it with cold hydrochloric acid. The pH was ensured to be less than pH 2 to obtain as much of the product as possible from the solution.
The benzilic acid rearrangement reaction could be understood from a molecular orbital point of view. After the nucleophilic attack by the OH- ion, the central C-C bond rotation brings the HOMO ( bond of the methylbenzyl group) into closer proximity to the LUMO of the tetrahedral intermediate. The LUMO is a linear combination of two * MOs of the carbonyl groups giving a set of bonding and antibonding MOs. Combination of these MOs in-phase produces a 4-electron system without any nodes and this is the LUMO which interacts with the pair of electrons from the HOMO. As the HOMO and the LUMO approach each other with the orbitals in-phase, the transition state which forms consists of 6 electrons and this is an aromatic system as it follows Huckel’s rule of 4n+2 whereby n = 1. The process is depicted as shown below:
As shown in the diagram, the bond of the methylbenzyl group acts as the HOMO to attack the in-phase bonding LUMO of the C=O * MOs. The equivalent transition state is shown on the far right side of the figure. As realigning the orbitals to ensure that they are in-phase with each other requires a relatively high amount of energy, this is the rate-determining step of the reaction.
The reaction mixture was heated under reflux for half an hour to ensure that the benzilic acid rearrangement occurs. It could be noted that as the reaction proceeded, the mixture turned brown due to the formation of potassium 4,4’-dimethylbenzilate. This salt is soluble in water, hence it was dissolved in warm water with heating and stirring. The acid work-up was done using HCl in ice, which forms the p-tolilic acid from the salt. The low temperature was to quench the reaction, prevent the decomposition of the product as well as to precipitate out as much of the product as possible. There were globules of unreacted organic compounds and side products at this point in time which would be filtered off later. The crude yellow product obtained by suction filtration was recrytallized by dissolving in a minimal amount of boiling water upon which gravity filtration was done to remove the oily globules that were present as impurities. The filtrate was left to stand to allow crystallization to occur.
IR Analysis
Wavenumber / cm-1
|
Vibrational mode
|
Intensity
|
3405.5
|
O-H stretching (alcohol)
|
Strong and sharp
|
2923.3
|
O-H stretching (carboxylic acid)
sp3 C-H stretching
|
Weak and broad
|
1719.1
|
C=O stretching (carboxylic acid dimer)
|
Strong and sharp
|
1513.2
|
C=C stretching
|
Weak and sharp
|
1256.8
|
C-O stretching (carboxylic acid dimer)
|
Medium and sharp
|
1173.1
|
C-O stretching (carboxylic acid monomer)
|
Medium and sharp
|
1068.4
|
C-O stretching (alcohol)
|
Strong and sharp
|
817.9
|
Aromatic OOP bending (para-substituted)
|
Strong and shrap
|
From the IR analysis, the peak at 3405.5 cm-1 was due to the O-H stretch of the alcohol’s hydroxyl functional group. The peaks at 1256.8 and 1173.1 cm-1 which corresponds to C-O stretching provides further evidence of the presence of an alcohol. The peak at 1719.1 cm-1 was assigned to the C=O stretch, which is part of the carboxylic acid functional group when the molecule is in its dimeric form. Monomeric carboxylic acids absorb at higher wavenumbers of around 1760 – 1730 cm-1. There are three C-O stretches in total. Two of them at 1256.8 cm-1 and 1173.1 cm-1 are due to the carboxylic acid dimeric and monomeric forms respectively. The C-O stretch at a lower wavenumber of 1068.4 cm-1 was due to the C-O of the alcohol. The dimeric form of the carboxylic acid tends to be present in the solid state and the IR was indeed done using a KBr disc. This dimerization also weakens the C=O bond and lowers its stretching force constant k, resulting in the lowering of the carbonyl stretching frequency to around 1710 cm-1. The aromaticity of the compound was also shown by the OOP bending at 817.9 cm-1 and this peak corresponds to the para-substituted benzene rings in the product. Besides doing IR analysis, 1H and 13C NMR could be done also to ascertain the synthesis of the desired product.
Percentage Yield and Melting Point Determination
The experiment which consists of three parts – Part A, B and C – had percentage yields of 66.5%, 61.8% and 3.65% respectively, with an overall yield of 1.48%. The first yield is reasonable at 66.5% and could be due to the amount of 4,4’-dimethylbenzoin remaining in solution even after crystallization. Other possible reasons might be that initial exposure to light has caused some of the p-tolualdehyde to be oxidized to p-toluic acid which decreased the amount of the limiting reagent available. Meanwhile, the second yield is 61.8% which is also reasonable, and this is probably due to the efficient work of the Cu2+ catalyst which was regenerated continuously in the synthesis. In the last part of the experiment, however, yield was considerably low at only 3.65%. The low yield could be due to the reaction of 4,4’-dimethylbenzil to form side products like 4,4’-dimethylbenzhydrol or 4,4’-dimethylbenzophenone when the 4,4’-dimethylbenzilate was heated to dissolve it. Thus, less of the p-tolilic acid was crystallized out due to lesser amount of the 4,4’-dimethylbenzilate present in the reaction mixture. On hindsight, it might have been more appropriate to evaporate the solvent instead to generate a higher yield of the fine white powder. The overall percentage yield of the experiment is 1.48%. This overall yield is considerably low as it is dependent on the individual yields of the three parts of the multi-step synthesis.
The melting point of the p-tolilic acid was determined to be 126.80 – 127.50 oC, which is lower than the literature value of 128 – 129 oC. This may be due to the presence of impurities. Recrystallization might be carried out to purify the product further though this risks losing more products in the recrystallization step.
Conclusion
The multistep synthesis of p-Tolilic acid from p-Tolualdehyde was carried out in a sequence of three steps – part A, B and C – having the percentage yields of 66.5%, 61.8% and 3.65% respectively. The overall yield was calculated to be 1.48%. IR analysis gave evidence for the formation of the target molecule of p-Tolilic acid as seen by the characteristic peaks from the carboxylic and alcohol functional groups. The melting point was determined to be 126.80 – 127.50 oC.
References
1. Carl T. Wigal. Copper-Catalyzed Oxidation of Benzoin to Benzil. H. A. Neidig, 2000. Pg 1 – 12.
2. Carl. T. Wigal. Thiamine-Catalyzed Benzoin Condensation. H.A. Neidig, 2000. Pg 1 – 12.
3. Carl T. Wigal and Jerry Manion. Converting Benzaldehyde to Benzilic Acid: A Multistep Synthesis. H.A. Neidig, 2000. Pg 1 - 4.
4. Clayden, Greeves, Warren and Wothers. Organic Chemistry, Oxford University Press, 2006. Pg 987, 989 – 990.
5. Floyd L. James and Anthony L. Ippolito. Rearrangement of p,p’-Disubstituted Benzils. Miami University, Ohio. The Ohio Journal of Science 53 (1) : 31, January, 1953. Pg 1 – 6.
6. Jon Landis and R. Minard (Penn State Univ.). Thiamine Catalyzed Benzoin Condensation. Adapted from K. L. Williamson: Macroscale and Microscale Organic Experiments, 2nd Ed. 1994, Houghton Mifflin, Boston. Revised 2/22/02.
7. George D. Maier and David E. Metzler. Structures of Thiamine in Basic Solution, Department of Chemistry, Iowa State College. Pg 4386 – 4391.
Comments
Post a Comment