The lab report below was submitted as part of the coursework for
CM3291 Advanced Experiments in Organic and Inorganic Chemistry. Please do not plagiarise from it as
plagiarism might land you into trouble with your university. Do note
that my report is well-circulated online and many of my juniors have
received soft copies of it. Hence, please exercise prudence while
referring to it and, if necessary, cite this webpage.
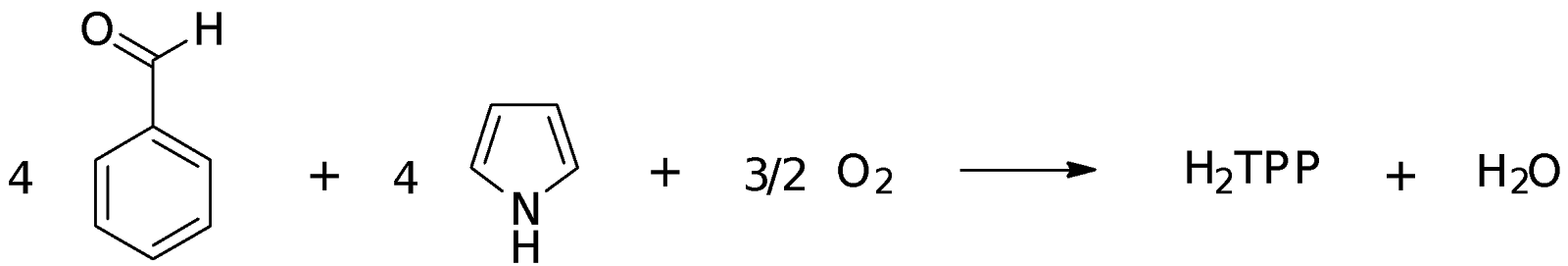

- Aim
The aim of this experiment is to first synthesize meso-tetraphenylporphyrin (H2TPP) which was used in turn to prepare Cu(TPP). H2TPP was analyzed using 1H NMR while both H2TPP and Cu(TPP) were analyzed with UV-Vis spectroscopy.
- Results & Calculations
Part A: Synthesis of meso-tetraphenylporphyrin (H2TPP)
Density of benzaldehyde: 1.0415 g ml-1
Vol. of benzaldehyde used: 1.70 ml
Mass of benzaldehyde used: 1.0415 x 1.70 = 1.77055 g
Molar mass of benzaldehyde: 7 x12.01 + 6 x 1.0079 + 16.00 = 106.12 g mol-1
Moles of benzaldehyde used: 1.77055 / 106.12 = 16.68 mmol
Density of pyrrole: 0.9670 g ml-1
Vol. of pyrrole used: 1.0 ml
Mass of pyrrole used: 0.9670 x 1.0 = 0.9670 g
Molar mass of pyrrole: 4 x 12.01 + 14.01 + 5 x 1.0079 = 67.09 g mol-1
Moles of pyrrole used: 0.9670 / 67.09 = 14.41 mmol
The mole ratio of benzaldehyde to pyrrole is 1:1. Hence, from the moles of the reagents calculated, pyrrole is the limiting reagent.
From the equation above, the mole ratio of pyrrole to H2TPP is 4:1. Hence, number of moles of H2TPP expected to be obtained is 14.41 / 4 = 3.603 mmol
Molar mass of H2TPP: 44 x 12.01 + 30 x 1.0079 + 4 x 14.01 = 614.72 g mol-1
Theoretical mass of H2TPP: 3.603 x 10-3 x 614.72 = 2.215 g
Actual mass of H2TPP obtained: 0.4584 g
Percentage yield of H2TPP: 0.4584 / 2.215 x 100% = 20.7 %
Preparation of solution for UV-Vis absorption spectrum
0.0056 g of H2TPP was added to 100 ml of chloroform in a 100-ml volumetric flask.
Concentration of H2TPP = (0.0056 / 614.72) / 100 x 1000 = 9.110 x 10-5 M
1 ml of this solution was then added to 99 ml of chloroform in another 100-ml volumetric flask.
Final concentration of H2TPP = (9.110 x 10-5 x 1) / 100 = 9.110 x 10-7 M
Using the Beer-Lambert law, whereby A = cl, the molar absorptivities for the various peaks in the uv-vis spectrum of H2TPP was calculated.
Table 1: Molar Absorptivities for H2TPP
/ nm
|
A
|
c / M
|
l / cm
|
/ M-1 cm-1
|
646.50
|
0.0050
|
9.110 x 10-7
|
1
|
5.488 x 103
|
591.00
|
0.0060
|
9.110 x 10-7
|
1
|
6.586 x 103
|
548.50
|
0.0083
|
9.110 x 10-7
|
1
|
9.111 x 103
|
514.50
|
0.0189
|
9.110 x 10-7
|
1
|
2.075 x 104
|
418.50
|
0.4835
|
9.110 x 10-7
|
1
|
5.307 x 105
|
273.50
|
0.0354
|
9.110 x 10-7
|
1
|
3.886 x 104
|
241.00
|
0.0344
|
9.110 x 10-7
|
1
|
3.776 x 104
|
Part B: Preparation of [Cu(TPP)]
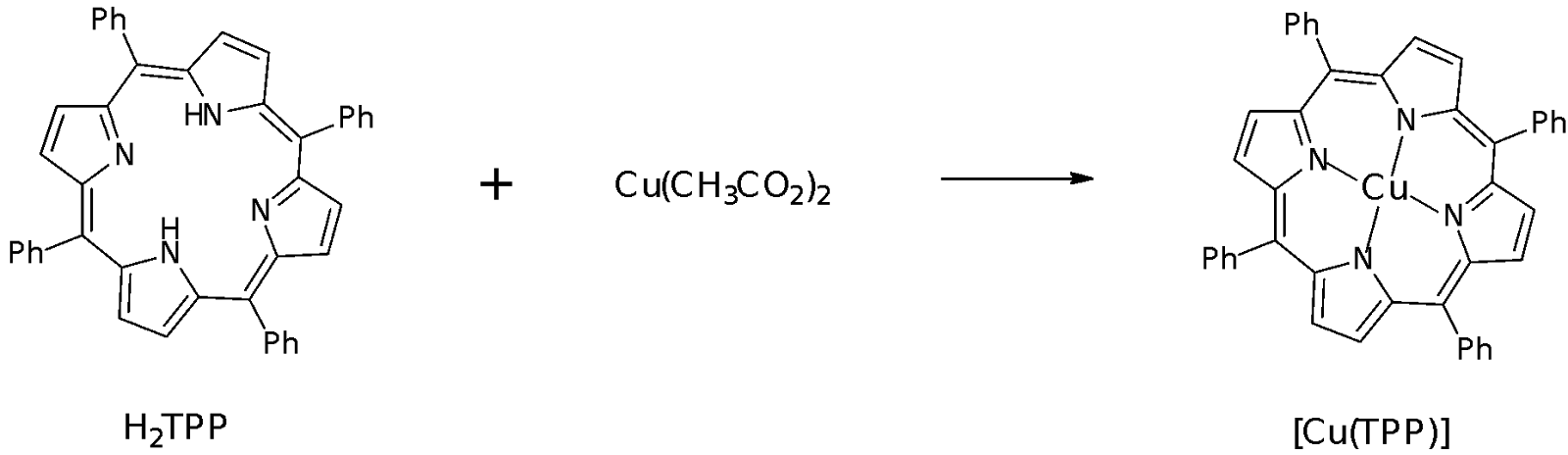
H2TPP + Cu(CH3CO2)2 Cu(TPP) + 2 CH3CO2H + H2O
Mass of H2TPP used: 0.1008 g
Moles of H2TPP used: 0.1008 / 614.72 = 0.1640 mmol
Mass of Cu(OAc)2 used: 0.1655 g
Molar mass of Cu(OAc)2: 199.65 g mol-1 (as labelled)
Moles of Cu(OAc)2 used: 0.1655 / 199.65 = 0.8290 mmol
As the mole ratio of H2TPP to Cu(OAc)2 is 1:1, from the moles of the reagents calculated, H2TPP is the limiting reagent.
Moles of Cu(TPP) expected: 0.1640 mmol
Molar mass of Cu(TPP): 63.55 + 44 x 12.01 + 28 x 1.0079 + 4 x 14.01 = 676.25 g mol-1
Theoretical mass of Cu(TPP): 0.1640 x 10-3 x 676.25 = 0.1109 g
Actual mass of Cu(TPP) obtained: 0.04028 g
Percentage yield of Cu(TPP): 0.04028 / 0.1174 x 100% = 36.3%
Preparation of solution for UV-Vis absorption spectrum
0.0065 g of H2TPP was added to 100 ml of chloroform in a 100-ml volumetric flask.
Concentration of Cu(TPP) = (0.0065 / 676.25) / 100 x 1000 = 9.612 x 10-5 M
1 ml of this solution was then added to 24 ml of chloroform in another 25-ml volumetric flask.
Final concentration of Cu(TPP) = (9.612 x 10-5 x 1) / 25 = 3.844 x 10-6 M
Using the Beer-Lambert law, whereby A = cl, the molar absorptivities for the various peaks in the uv-vis spectrum of Cu(TPP) was calculated.
Table 2: Molar Absorptivities for CuTPP
/ nm
|
A
|
c / M
|
l / cm
|
/ M-1 cm-1
|
539.50
|
0.0183
|
3.844 x 10-6
|
1
|
4.761 x 103
|
416.00
|
0.2632
|
3.844 x 10-6
|
1
|
6.847 x 104
|
276.00
|
0.0205
|
3.844 x 10-6
|
1
|
5.333 x 103
|
247.00
|
0.0221
|
3.844 x 10-6
|
1
|
5.749 x 103
|
213.00
|
0.0186
|
3.844 x 10-6
|
1
|
4.839 x 103
|
- Discussion
Part A: Synthesis of meso-tetraphenylporphyrin (H2TPP) (Reaction and mechanism)
In this experiment, H2TPP was synthesized using benzaldehyde and pyrrole. The reaction involves the condensation of four molecules each of benzaldehyde and pyrrole using propanoic acid as the catalyst and solvent. Propanoic acid is a weak acid that has a pKa of 4.87. A strong acid is not used as it might lead to the dimerization of pyrrole instead: 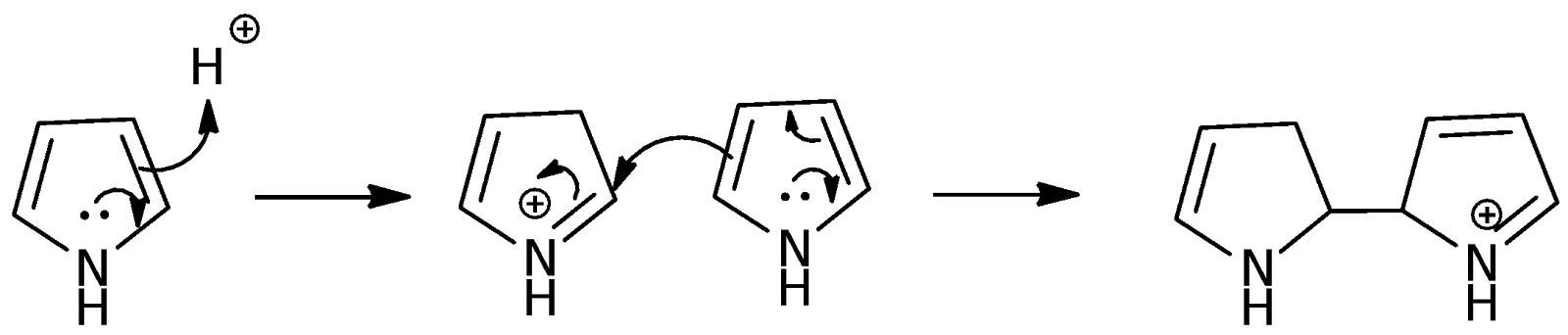
The propanoic acid was also heated up to ensure that it would react readily once the reagents are placed into the round-bottomed flask. The high starting reflux temperature would also reduce the formation of the pyrrole polymers as the pyrrole is used up more readily in the H2TPP synthesis reaction, as the heat provides the energy to overcome the activation energy barrier.
The mechanism of the H2TPP synthesis reaction involves a series of repeated pyrrole and benzaldehyde condensation reactions with pyrrole as the nucleophile and benzaldehyde as the electrophile. The propanoic acid used increases the electrophilicity of the benzaldehyde such that it becomes more likely for the pyrrole to attack it instead of undergoing dimerization. The full mechanism is shown below:
In the first step of the mechanism, the oxygen of the carbonyl group in benzaldehyde accepts a proton which makes the group more electrophilic. The pyrrole then acts as a nucleophile and attacks the electrophilic carbonyl carbon from the -position of the pyrrole. The attack is at the -position instead of the -position because the deprotonation following the attack at the -position leads to the formation of a quaternary carbon which is very unlikely. For the -position, subsequent deprotonation of removes the positive charge on the nitrogen in pyrrole and re-establishes the aromaticity of the pyrrole. In the pyrrole ring, there are 4 electrons and 2 lone pair electrons which makes a total of 6, thereby following Huckel’s rule of 4n + 2 where n=1. The next step involves the hydroxyl group being protonated with the loss of a water molecule to generate a carbocation. This facilitates the addition of another pyrrole molecule to the carbocation. These steps are repeated to eventually form a nonconjugated macrocycle. The oxidation of this macrocycle by oxygen then generates the fully conjugated porphyrin ring. The H2TPP is insoluble in propanoic acid, which causes purple crystals to appear upon cooling. The crystals were washed with methanol to remove the propanoic acid and the excess reagents of benzaldehyde and pyrrole.
The product was then sent for 1H NMR analysis of which the results have been tabulated on the next page:
1H NMR Analysis of H2TPP (in CDCl3)
Table 3: 1H NMR Assignment for H2TPP
/ ppm
|
Multiplicity
|
Integration
|
No. of protons
|
Assignment
|
-2.764
|
Singlet
|
0.46
|
2
|
Ha
|
7.768
|
Multiplet
|
3.00
|
12
|
Hd, He
|
8.216
|
Multiplet
|
1.91
|
8
|
Hc
|
8.849
|
Singlet
|
1.86
|
8
|
Hb
|
1.539
|
-
|
-
|
H2O
| |
7.257
|
-
|
-
|
CHCl3
|
For H2TPP, protons found in the centre of the molecule are shielded due to the anisotropic effect from the ring current in the -system. As such, the Ha protons have a low chemical shift of -2.764 ppm. They are also not coupled as there are no neighbouring protons within three bond lengths away; hence the signal shows up as a singlet. Meanwhile, on the periphery of the porphyrin ring, the protons found here are deshielded due also to the anisotropic effect of the conjugated porphyrin system. These Hb protons are thus found at a high chemical shift value of 8.849 ppm and are also not coupled as there are no neighbouring protons within three bond lengths away. The Hb protons are all chemically equivalent due to delocalization of the -electrons in the porphyrin ring system.
The signal at 8.216 ppm has been identified as Hc. The chemical shift of this signal is less than that for Hb because the Hc protons lie further away from the porphyrin ring than Hb, hence are correspondingly less deshielded. This leads to a lower chemical shift observed as compared to Hb. The signal on the NMR spectrum looks like a doublet due to ortho-coupling with Hd. However, on closer inspection, the signal resembles a doublet of doublets as the Hc protons are actually not chemically equivalent. This means that the ortho protons on each of the benzene groups actually also couple with each other. The end result is a multiplet signal which is complicated by second-order spectral effects. A higher resolution NMR instrument would be required to distinguish the signals more clearly.
The signal at 7.768 ppm has been identified to be Hd and He. Hd should be a doublet of doublets due to coupling with the Hc and He protons while He should be a triplet due to coupling with 2 Hd protons. However, their respective signals have overlapped and hence, could not be distinguished from each other unless a higher resolution instrument is used. The chemical shift of this signal is even lower than that of Hc because these protons are further away from the porphyrin aromatic system, and hence experience a lower deshielding anisotropic effect. Comparison of the integration ratios serves to check that the assignment is proper, as there are 12 protons in total for Hd and He. The NMR analysis done thus shows that H2TPP was indeed synthesized.
There were other peaks present at 1.539 ppm and 7.257 ppm. These peaks were due to H2O and CHCl3 respectively. The H2O was present due to the condensation reaction of benzaldehyde and pyrrole, with some of the water remaining behind in the product. The CHCl3 peak is present due to the use of CDCl3 as the solvent for the NMR sample. As the solvent is not entirely deuterated, some of the CDCl3 is present as CHCl3 instead, which gives rise to the peak at 7.257 ppm. This sharp peak is useful as it becomes the reference signal from which other peaks are calibrated. It could be noted that there are other unassigned signals in the NMR spectrum and these were most likely due to impurities like meso-tetraphenylchlorin which was formed as a by-product of the reaction.
Part B: Preparation of [Cu(TPP)] (Reaction and purification)
In the preparation of Cu(TPP), DMF was used as a polar aprotic solvent to solvate the reagents as well as to deprotonate the H2TPP in order for the TPP to complex with the Cu2+ metal ion. The Cu2+ ion is formed from the solvation of hydrated copper (II) acetate. The TPP is a tetradentate ligand that binds to the Cu2+ ion at the four N atoms present in the porphyrin ring. Cu(OAc)2 was added in access to make it more likely that the Cu2+ is able to coordinate to the TPP ring.
Extraction was done with distilled water and dichloromethane added to the reaction mixture. Cu(TPP) was extracted into the organic layer while the rest of the reaction mixture consisting of unreacted copper (II) acetate and DMF solvent went into the aqueous layer. The combined organic extracts were rinsed once more with distilled water to get rid of DMF solvent which is miscible with the organic layers as well. After this, the solvent was removed by rotary evaporation to obtain the crude product of Cu(TPP).
The crude product obtained was purified using column chromatography with silica gel as the stationary phase. Contaminants present include meso-tetraphenylchlorin which was formed by a reduction of the H2TPP in part A. The mobile phase of 1:1 hexane/toluene mixture was used to elute out the purified Cu(TPP). The column was prepared by first stuffing a cotton plug at the base of the column, upon which a layer of sea sand was placed on top of it. Silica gel in hexane/toluene solvent was poured down the column to make up a stationary phase height of about 25cm. Care was taken to ensure that there were no air bubbles trapped in the column, with the column being tapped periodically. Sand was then added on top of the silica gel to protect the stationary phase such that addition of the sample would not disrupt the packed layers of silica gel. The crude product was dissolved in hexane/toluene solvent upon which it was poured gently down the column. It was allowed to flow down the column before hexane/toluene solvent was added on top to push the eluent down by gravity. As the sample consists of components with different affinities, the more polar substances have more interactions with the silica gel and hence move more slowly down the column. This was seen as a dark patch of red at the top of the column. The Cu(TPP) is less polar and hence is more easily eluted out, and this was observed visibly as a lighter red colour which flowed down the column. This was collected and then placed in the rotary evaporator to remove the solvent, and obtain the pure Cu(TPP) product.
Analysis of yield
The yield for the synthesis of H2TPP is 20.7% which is comparable to the literature values put the yield at around 20% depending on numerous factors like acidity, the solvent used, temperature and the availability of atmospheric oxygen. The yield is low due to a number of reasons. Firstly as the mechanism shows a multi-step process in the assembly of the large molecule, with each step being reversible, the final product has an overall lower tendency to form. Also, some of the macrocycles might not have been oxidized to H2TPP or some of the H2TPP might have been reduced in situ to meso-tetraphenylchlorin. This leads to the decrease in the overall yield of H2TPP.
Meanwhile, for the synthesis of Cu(TPP), the yield is 36.3%. This is a relatively significant yield which could mean that the reaction progressed well due to the addition of excess amounts of Cu(OAc)2. This prompted the complexation reaction between the Cu2+ ions and the TPP ligand. However, the high yield might be due also to the presence of impurities which might be because the separation by column chromatography caused the elution of some of the undesired compounds along with the product. An alternative purification method involves the use of 2,3-Dichloro-5,6-Dicyanobenzoquinone (DDQ) which would oxidize the chlorine to yield H2TPP. This would be a more efficient process for purification though it requires additional steps to remove the excess quinine and quinol chemically. After which pure H2TPP could be crystallized out.
UV-Vis Spectrum Analysis
Both H2TPP and Cu(TPP) were analysed using UV-Vis spectroscopy. UV-Vis spectroscopy is based on the principle of electronic transitions whereby photons are absorbed corresponding to the difference in energy levels of two molecular orbitals. Beer-Lambert’s law of A = cl applies and the molar absorptivities for the respective peaks have been tabulated in the results section. The solutions for UV-Vis spectroscopy were diluted to about ~10-7 M to ensure a more accurate determination of the spectrums. If the concentration is too high, the absorbance would be underestimated as molecules lying along the same path would absorb less UV-Vis radiation than expected. This is because the molecules in front would block those that are behind.
From the results obtained, there are two regions of interest which are known as the Soret (~410 - 440 nm) and the Q bands (~450 - 700 nm). Both Soret and Q bands arise from to * transitions and can be explained using the Gouterman four orbital model that describes the HOMO and LUMO orbitals involved in the transitions. The Soret band involves a strong transition from the ground state to the second excited state while the Q band involves a weak transition from the ground state to the first excited state.
According to the Gouterman model, the absorption bands in porphyrin systems arise from transitions between two HOMOs and two LUMOs. The identity of the metal centre and the substituents on the ring can affect the relative energies of the transitions. As shown in the diagram below, the HOMO consists of the a1u and a2u orbitals while the LUMO comprises a set of degenerate eg orbitals. Transitions from the HOMO to the LUMO results gave rise to two excited singlet states of 1Eu character. The Soret band corresponds to the transition of eg (*) ← a1u () while the Q band corresponds to the transition of eg (*) ← a2u (). The mixing of these two singlet 1Eu states creates a state of higher energy with greater oscillator strength and a state of lower energy with less oscillator strength. Transition from the ground to higher energy 1Eu state gives rise to the Soret band while the transition from the ground state to the lower energy 1Eu state gives rise to the Q band. 
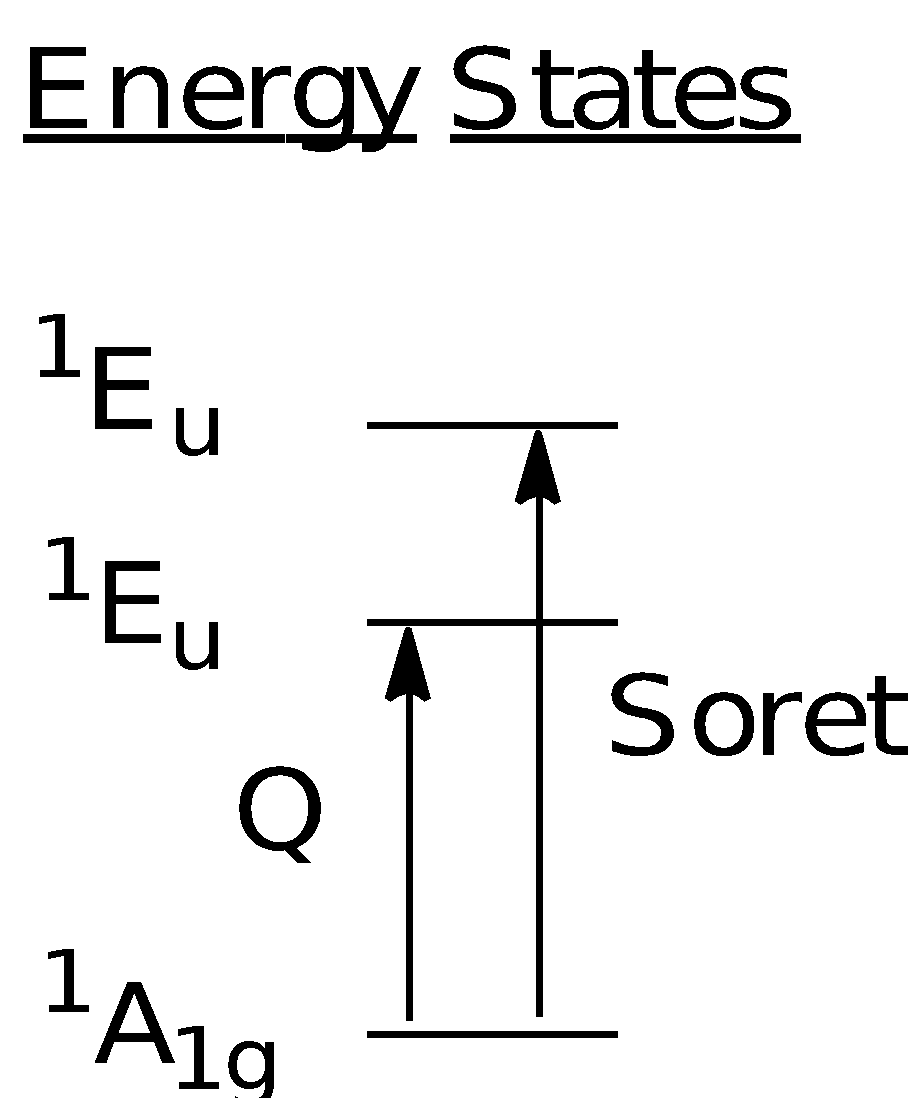
The intensities of the Soret bands are higher than that of the Q bands as observed in the spectrums obtained. The difference in intensity is due to the degree of “allowedness” of the transitions. Transitions occurring in the Soret band are more strongly allowed (∆ml = ±1) as compared to forbidden transitions occurring in the Q band (∆ml ≠ ±1), which explains the intensity difference. The results for the uv-vis spectroscopy have been tabulated as shown below:
Soret bands
|
Q bands
| |||||
/ nm
|
/ M-1 cm-1
|
Transition
|
/ nm
|
/ M-1 cm-1
|
Transition
| |
H2TPP
|
418.50
|
5.307 x 105
|
eg (*) ← a1u ()
|
646.50
|
5.488 x 103
|
eg (*) ← a2u ()
|
591.00
|
6.586 x 103
| |||||
548.50
|
9.111 x 103
| |||||
514.50
|
2.075 x 104
| |||||
Cu(TPP)
|
416.00
|
6.847 x 104
|
eg (*) ← a1u ()
|
539.50
|
4.761 x 103
|
eg (*) ← a2u ()
|
From the Soret bands of the respective compounds, there is a blue shift of 2.50 nm occurring for the Cu(TPP) when compared against the H2TPP. This is because the d9 Cu2+ ion in Cu(TPP) has filled d orbitals which interact significantly with the porphyrin * orbitals via metal to ligand -backbonding. This results in an increased porphyrin to * energy separation causing the electronic absorptions to undergo hypsochromic (blue) shifts. It could also be noted that the phenyl groups and the porphyrin rings were not in the same plane, hence the conjugated effect between the phenyl substitutes and the porphyrin was relatively weak. Thus, there was not much effect of the phenyl substitutes on the Soret bands.
Meanwhile, for the Q bands, there are 4 bands for the H2TPP whereas there’s only one Q band for the Cu(TPP) molecule. This is because the coordination of the metal to the porphyrin molecule increases the symmetry and thus, the number of Q bands decreases. H2TPP has a two-fold symmetry of D2h while Cu(TPP) has a four-fold symmetry of D4h.
There were other peaks observed in the near UV region (~200 - 300 nm) and these bands could possibly be attributed to the absorptions of higher lying to * transitions occurring between orbitals with greater energy gaps than that of the HOMO-LUMO.
From the UV-Vis spectra obtained, it could be used as evidence that the corresponding compounds synthesized were H2TPP and Cu(TPP). The spectrum also explains the colour of the compounds. For H2TPP, it is bluish-purple in colour while Cu(TPP) is red in colour. This is because Cu(TPP) does not absorb in the 600 – 700 nm (red) region unlike H2TPP. Thus, the Cu(TPP) appears more reddish as compared to H2TPP.
- Conclusion
The compounds H2TPP and Cu(TPP) were synthesized with yields of 20.7% and 36.3% respectively. 1H NMR spectrum results confirm that H2TPP was synthesized. As for the uv-vis spectrum results, H2TPP had a Soret band at 418.50 nm with a molar absorptivity of 5.307 x 105 M-1 cm-1 while Cu(TPP) had a Soret band at 416.00 nm with a molar absorptivity of 6.847 x 104 M-1 cm-1. The Soret band for Cu(TPP) was blue shifted from the H2TPP by 2.5 nm due to the backbonding effect. H2TPP also showed four Q bands as compared to Cu(TPP) which only had one Q band and this was due to the lower-order symmetry of H2TPP.
- References
Bjorn Roos and Marianne Sundbom. A Theoretical Investigation of the Electronic Structure and Excited States of Copper Porphin. Journal of Molecular Spectroscopy 36 (1970), pp 8 – 25.
JJ Weaver. Electronic Structures And Absorption Spectra. Caltech. Chapter 3, pp 35 – 45.
Kathleen Rousseau. A Purification of Meso-tetraphenylporphyrin. Department of Chemistry, Harvard University. Tetrahedron Letters No. 48, pp 4251 – 4254 (1974). Pergamon Press, UK.
M. Prushan. Electronic Spectroscopy of free base porphyrins and metalloporphyrins. La Salle University, 2005, pp 1 – 11.
Minbo Lan, Hongli Zhao, Huihui Yuan, Chengrui Jiang, Shaohua Zuo, Ying Jiang. Absorption and EPR spectra of some porphyrins and metalloporphyrins. Dyes and Pigments 74 (2007), pp 357 – 362.
Wengqi Zheng, Ning Shan, Lianxiang Yu, Xingqiao Wang. UV-visible, fluorescence and EPR properties of porphyrins and metalloporphyrins. Dyes and Pigments 77 (2008), pp 153 – 157.
what is the reference of total mechanism???
ReplyDeleteDid this so long ago that I don't remember!
Deletehttp://www.uvm.edu/~mcase/courses/chem143/porphyrin.pdf
ReplyDelete