The lab report below was submitted as part of the coursework for CM1101 Basic Analytical Chemistry. Please do not plagiarise from it as plagiarism might land you into trouble with your university. Do note that my report is well-circulated online and many of my juniors have received soft copies of it. Hence, please exercise prudence while referring to it and, if necessary, cite this webpage.
Abstract
In this experiment, the isolation of two plant pigments, with different polarities, namely chlorophyll and β-carotene, by column chromatography will be carried out. After separation and isolation, the two chemicals would be identified by Ultraviolet-visible (UV/VIS) Absorption Spectroscopy. Due to the conjugated system of double bonds in both chemicals, they absorb light of longer wavelengths and this absorption is reflected in the spectrum obtained. The results, as seen from the spectrum, show that chlorophyll and β-carotene absorbs different range of wavelengths of light.In conclusion, from the presented data, it is proven that chlorophyll and β-carotene work together to maximize photon absorption to increase the rate of photosynthesis.
Introduction
The experiment aims to isolate chlorophyll and β-carotene and identify them by their different absorption wavelengths through the use of UV/VIS Absorption Spectroscopy.
UV/VIS Absorption spectroscopy uses the particle in a box theory to measure the absorption wavelengths of two compounds. The theory indicates that an electron can never be at rest and would always possess energy and this can be represented by Heisenberg’s Uncertainty Principle, ΔxΔp≥h/4π. When a photon of the right wavelength is absorbed by an electron in the molecules, the electron is excited from a lower energy level to a higher energy level. Using the formula, E=hc/λ, the energy difference between the two energy levels can be calculated.
β-carotene is a non-polarhydrocarbon molecule as it contains mainly C-C, C=C and C-H bonds.Chlorophyll is a polar molecule as it carries ionic charges, on the Mg2+ and amide ions, and has N and O atoms with lone pair of electrons. A non-polar solvent, hexane, was added to elute β-carotene from the chromatography column first. β-carotene, having similar intermolecular dispersion forces as hexane, will dissolve in hexane and be eluted. After the complete elution of β-carotene, apolar solvent, ethyl acetate, was added to elute chlorophyll, according to the same principle aforementioned. It is possible to separate the compounds, β-carotene and chlorophyll, due to their different polarities.
A conjugated system of π electrons is present in the molecules. This system, called a chromophore, forms the light-absorbing portion of a molecule, absorbs light of certain wavelengths and causes a compound to have colour. When electrons are excited from a lower energy level to a higher energy level, they absorb photons with wavelengths that correspond to the difference in the energy levels. The wavelengths of light that are displayed in a UV/VIS spectrum are the absorption peaks reflected in the absorption spectrum.
After the electron in the chlorophyll has been excited by the absorption of a photon, it will undergo a chain reaction in the electron transport chain and finally react with carbon dioxide to produce a carbohydrate and oxygen, according to the photosynthesis equation, CO2+H2O+hv→(CH2O)x+O2.β-carotene is a secondary electron-carrier molecule in plants and act as a ‘backup’ carrier for chlorophyll.
Experimental
1. Preparation of Sample
The 5.0 g of spinach leaves were treated with 15mL of ethanol to remove the water on the leaves. The plant sample was then taken out and pressed dry on a paper towel to remove all the water and ethanol. Following which, 20mL of dicholoromethane was added and the leaves were grinded to extract the chlorophyll and β-carotene. The mixture was then filtered to remove the leaves and the resultant filtrate heated until about 1mL remained.
2. Column chromatography
The chromatography column was assembled by placing a small cotton plug in the bottom of the column, followed by pouring of around 2mm of sea sand over. This prevented the solid alumina from entering the stopcock. Hexane was added to 10g of alumina to produce a slurry that was added to the top of the sea sand. Excess hexane was drained out till only 0.5cm of hexane covers the alumina. 1mL of the plant extract was carefully transferred into the column. Hexane was added and the eluted yellow β-carotene collected in a dry test tube. Then, ethyl acetate was added slowly to elute the green chlorophyll layer. Collect the green chlorophyll layer in another dry test tube.
3. Ultraviolet-visible Spectroscopy of Chlorophyll and β-Carotene
3 separate samples of chlorophyll, β-carotene and hexane were prepared. The hexane was blanked with the UV/VIS spectrometer to ensure that the resultant absorbance reflected in the spectrum belong wholly to either chlorophyll or β-carotene and is not affected by the absorbance of impurities in hexane. The two spectrum of chlorophyll and β-carotene were then printed out for analysis.
Results
After isolation of the compounds, the solution containing chlorophyll was found to be green and the solution containing β-carotene is yellow. Setting the range of the ultra-violet spectrometer to be 400.0nm to 800.0nm, the chlorophyll and β-carotene extracted has peaks as shown below.
Discussion
Accounting for the observed absorption spectrum of Chlorophyll and β-carotene
The theoretical values for the absorbance of α-chlorophyll and β-chlorophyll have approximate absorption peaks at 430 nm and 662 nm, and at 453 nm and 642 nm respectively (see Figure 1). The expected spectrum for the chlorophyll sample should have 4 distinct peaks, and the experimentally recorded sample have 3 distinct peaks that correspond with the theoretical peaks and 1 unexpected peak at 728nm. From the spectrum, the unexpected peak at 728nm is insignificant and can be attributed to poor data processing by the spectrometer. Also, despite a clear peak in the spectrograph, the data sheet failed to process a visible and significant absorbance peak at 430nm.
The experimental result for the absorption maxima of β-carotene is 448nm and falls within the expected theoretical absorption range of 440-450nm (see Figure 2). There should be another peak at approximately 470nm, but it was not reflected on the data sheet. This is due to the poor scaling of the graph which causes the overlap of the two peaks. There is an unexpected but minor absorption peak at 670nm in the experimental spectrum. This is because there is contamination of the β-carotene sample with some residual chlorophyll, which has absorption peaks at around 670nm.
Hence, the two compounds are successfully isolated.
Discussion on the procedures taken
Ethanol was used to remove water, wax or other substances that may be present on the surfaces of the leaves. These surface materials are removed as they may be insoluble in dichloromethane and therefore, prevent dichloromethane from dissolving chlorophyll and β-carotene. As chlorophyll is soluble in ethanol, the leaf is only immersed in ethanol for a short period of time. This reduces the probability of chlorophyll dissolving out and improves the accuracy of the experiment. β-carotene is insoluble in ethanol and thus, both chemicals have to be dissolved into a common solvent, ethyl acetate, to carry out the column chromatography. Every step of the experiment should be carried out in the fume hood as dichloromethane is highly volatile and an inhalation hazard. A small cotton plug is positioned at the bottom of the column to prevent alumina from being washed out of the column.
After the addition of hexane and alumina to the column, any air bubbles should be displaced. This ensures the even distribution and packing of the stationary phase so that the samples can travel evenly down the column and separation of chlorophyll and β-carotene can take place accurately.
The solvent layer should be added carefully and slowly to avoid uneven channeling. Also, the solvent layer should always completely cover the solid layer of alumina to prevent air bubbles from forming, which may lead to poor separation.
Alumina is an adsorbent stationary phase for column chromatography. It affects the flow of solvent through the column. Any polar solvents present will bind closely to alumina and flow less readily through stationary phase of the column. Thus, the polarity of the solvent directly affects the rate of elution of chlorophyll and β-carotene. Often, a series of increasing polar solvent systems are used to elute a column. Firstly, a non-polar solvent like hexane is used to elute the less polar β-carotene. Once β-carotene is eluted, a more polar solvent mixture of hexane-acetone is used to elute the more polar chlorophyll. Acetone is more polar than hexane as it has a net dipole moment.
The nature of the solvent and the order of adding the solvent must be considered. The less polar solvent, hexane, should be used first. This ensures that only the less polar β-carotene will travel rapidly down the column. Once β-carotene is eluted, a polar solvent is added to hasten the elution of the more polar chlorophyll. If the most polar solvent is used first, both chemicals will travel rapidly down the chromatography column and no separation will take place.
Also, the strength of the pump used must be moderate. If it is too fast or too slow, the isolation of chemicals will become inaccurate.
Only a slight volume of spinach pigment in dichloromethane was added to the column. This is to prevent dichloromethane, a polar solvent, from disrupting with the separation results.
A separate sample of hexane was blanked with the UV/VIS spectrometer to ensure that the absorbance of the impurities present in hexane would be factored in and eventually cancelled from the resultant spectrum of chlorophyll and β-carotene.
Accounting for why UV/VIS was absorbed
Only π –> π*, n –> π* and n –> σ* usually produce absorption in the UV/VIS region. Thus, only molecules with n or π electrons give characteristic UV/VIS spectra.
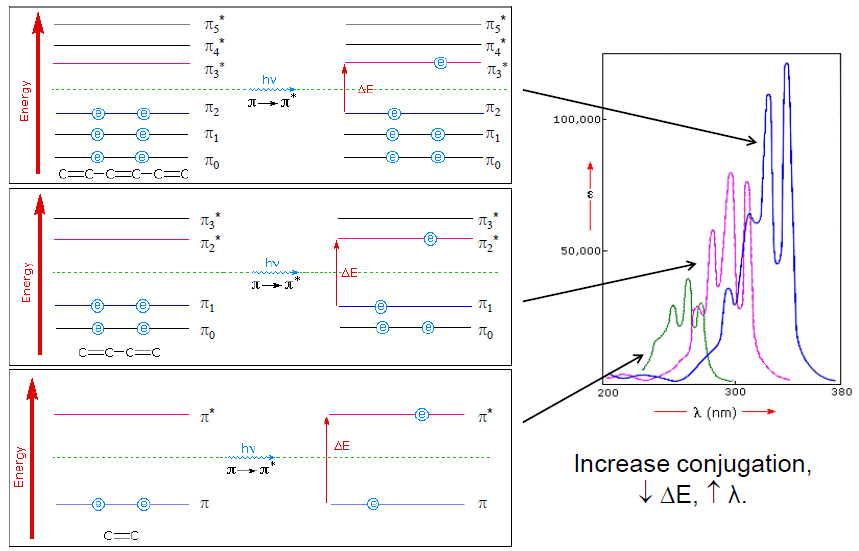
Alternating single and double bonds is called conjugation. This extended conjugated system leads to a significant π-electron delocalization. This extended π-system gains its stability by being planar as it allows all the p AOs to overlap efficiently.
This causes the energy gap between π and π* to decrease (see Figure 3) and shifts the absorbance of the molecule to longer wavelengths. It requires a photon with lesser energy and longer wavelength to excite an electron from π –> π*.
The incorporation of an atom with lone pairs of electrons can cause a shift of absorbance to longer wavelengths as n –> π* energy gap is smaller than π –> π* gap. Chlorophyll absorbs photons of longer wavelengths than β-carotene and has additional peaks at around 642 nm as it contains N and O atoms with lone pair of electrons.
Discussion on the reliability of results
Spinach pigments are organic molecules and are affected by physical conditions such as temperature, air and light. If not protected from these conditions, they can undergo hydrolysis, atmospheric oxidation and other reactions. The extracted sample of spinach pigment was heated in order to concentrate the leaf pigments and remove excess dichloromethane. This could lead to more peaks than expected. A better alternative would be to pass a gentle steam of air over the solution in a fume hood to slowly evaporate it.
Leaf pigments contain not only chlorophyll and β-carotene. It might contain other compounds such as Xanthophylls, Pheophytin A and Pheophytin B. This may result in small anomalous peaks contaminating the samples and interfere with the collected data. For example, both the absorption peaks of Xanthophylls and β-carotene are around 400 to 500 nm. Therefore, different molecules with similar conjugated systems may have similar UV/VIS spectrum.
The solvents, acetone and hexane, do not absorb UV/VIS spectrum due to the absence of a conjugated system of π bonds and atoms with lone pairs of electrons. Thus, theoretically, they should have no effect on the spectra of chlorophyll and β-carotene. However, should there be impurities in the solvents, the reliability of the results can be improved by using hexane as a blank for β-carotene and ethyl acetate as a blank for chlorophyll when doing the UV/VIS spectroscopy, instead of using hexane as a blank for both chemicals. This is because the main solvent for β-carotene is hexane and the main solvent for chlorophyll is ethyl acetate. Thus, using the respective solvents as blanks would allow for the more accurate correction of absorbance data by the spectrometer, if there are impurities in the solvent.
Instead of comparing the absorption peaks of the resultant spectra to a table of known functional groups and their associated absorbance then piecing the information before postulating about the likely structure of the molecules, the entire resultant spectra of the respective molecules was compared to their standard absorption spectra. The first approach will lead to inaccurate results as the table of absorbance values are average values and not specific to molecular species. The latter approach used in this experiment is appropriate as it will lead to more accurate results.
The cuvettes must be wiped dry and have surfaces free from fingerprints. Any surface dirt might cause the scattering of light during the UV/VIS spectroscopy. This will be misinterpreted by the spectrometer as absorbance of photons and the resultant spectra might have anomalous absorbance peaks.
The sample being used for UV/VIS spectroscopy should, ideally, be dilute and homogeneous. If the sample is concentrated, light from the light source may be scattered by the densely packed particles.Likewise, this scattering of light will be misinterpreted as absorbance by the spectrometer and may lead to inaccurate results.
Conclusion
Chlorophyll and β-carotene, having different polarities, are separated and isolated by column chromatography. Due to the conjugated system of double bonds present in both molecules, the two molecules absorb photons of longer wavelengths in the region of UV/VIS spectrum. By comparing the peak absorptions to the standard absorption peaks of the respective molecules, UV/VIS spectroscopy was used to distinguish between chlorophyll and β-carotene.The absorption peaks of chlorophyll is experimentally determined to be 456, 664 and 610 nm while that of β-carotene is 448 and 670 nm.
References
[1] http://en.wikipedia.org/wiki/File:Chlorophyll_ab_spectra2.PNG
[2] http://www.chm.bris.ac.uk/motm/carotene/absorp~3.gif
[3] http://en.wikipedia.org/wiki/Ultraviolet%E2%80%93visible_spectroscopy
[4] http://www.snypa.co.uk/OSR/UV/instrument.php
[5] http://www.scribd.com/doc/6379245/UV-SPECTROPHOTOMETER-THEORY
[6] http://www.uwgb.edu/heuerc/2D/ColorTerms.html
[7] http://141.161.23.43/S00/handout/spectrometer.htm
[8] http://www.chemlin.net/chemistry/uv_vis_spectroscopy.htm

Abstract
In this experiment, the isolation of two plant pigments, with different polarities, namely chlorophyll and β-carotene, by column chromatography will be carried out. After separation and isolation, the two chemicals would be identified by Ultraviolet-visible (UV/VIS) Absorption Spectroscopy. Due to the conjugated system of double bonds in both chemicals, they absorb light of longer wavelengths and this absorption is reflected in the spectrum obtained. The results, as seen from the spectrum, show that chlorophyll and β-carotene absorbs different range of wavelengths of light.In conclusion, from the presented data, it is proven that chlorophyll and β-carotene work together to maximize photon absorption to increase the rate of photosynthesis.
Introduction
The experiment aims to isolate chlorophyll and β-carotene and identify them by their different absorption wavelengths through the use of UV/VIS Absorption Spectroscopy.
UV/VIS Absorption spectroscopy uses the particle in a box theory to measure the absorption wavelengths of two compounds. The theory indicates that an electron can never be at rest and would always possess energy and this can be represented by Heisenberg’s Uncertainty Principle, ΔxΔp≥h/4π. When a photon of the right wavelength is absorbed by an electron in the molecules, the electron is excited from a lower energy level to a higher energy level. Using the formula, E=hc/λ, the energy difference between the two energy levels can be calculated.
β-carotene is a non-polarhydrocarbon molecule as it contains mainly C-C, C=C and C-H bonds.Chlorophyll is a polar molecule as it carries ionic charges, on the Mg2+ and amide ions, and has N and O atoms with lone pair of electrons. A non-polar solvent, hexane, was added to elute β-carotene from the chromatography column first. β-carotene, having similar intermolecular dispersion forces as hexane, will dissolve in hexane and be eluted. After the complete elution of β-carotene, apolar solvent, ethyl acetate, was added to elute chlorophyll, according to the same principle aforementioned. It is possible to separate the compounds, β-carotene and chlorophyll, due to their different polarities.
A conjugated system of π electrons is present in the molecules. This system, called a chromophore, forms the light-absorbing portion of a molecule, absorbs light of certain wavelengths and causes a compound to have colour. When electrons are excited from a lower energy level to a higher energy level, they absorb photons with wavelengths that correspond to the difference in the energy levels. The wavelengths of light that are displayed in a UV/VIS spectrum are the absorption peaks reflected in the absorption spectrum.
After the electron in the chlorophyll has been excited by the absorption of a photon, it will undergo a chain reaction in the electron transport chain and finally react with carbon dioxide to produce a carbohydrate and oxygen, according to the photosynthesis equation, CO2+H2O+hv→(CH2O)x+O2.β-carotene is a secondary electron-carrier molecule in plants and act as a ‘backup’ carrier for chlorophyll.
Experimental
1. Preparation of Sample
The 5.0 g of spinach leaves were treated with 15mL of ethanol to remove the water on the leaves. The plant sample was then taken out and pressed dry on a paper towel to remove all the water and ethanol. Following which, 20mL of dicholoromethane was added and the leaves were grinded to extract the chlorophyll and β-carotene. The mixture was then filtered to remove the leaves and the resultant filtrate heated until about 1mL remained.
2. Column chromatography
The chromatography column was assembled by placing a small cotton plug in the bottom of the column, followed by pouring of around 2mm of sea sand over. This prevented the solid alumina from entering the stopcock. Hexane was added to 10g of alumina to produce a slurry that was added to the top of the sea sand. Excess hexane was drained out till only 0.5cm of hexane covers the alumina. 1mL of the plant extract was carefully transferred into the column. Hexane was added and the eluted yellow β-carotene collected in a dry test tube. Then, ethyl acetate was added slowly to elute the green chlorophyll layer. Collect the green chlorophyll layer in another dry test tube.
3. Ultraviolet-visible Spectroscopy of Chlorophyll and β-Carotene
3 separate samples of chlorophyll, β-carotene and hexane were prepared. The hexane was blanked with the UV/VIS spectrometer to ensure that the resultant absorbance reflected in the spectrum belong wholly to either chlorophyll or β-carotene and is not affected by the absorbance of impurities in hexane. The two spectrum of chlorophyll and β-carotene were then printed out for analysis.
Results
After isolation of the compounds, the solution containing chlorophyll was found to be green and the solution containing β-carotene is yellow. Setting the range of the ultra-violet spectrometer to be 400.0nm to 800.0nm, the chlorophyll and β-carotene extracted has peaks as shown below.
[Figure1] Absorption spectrum of chlorophyll
|
[Figure 2] Absorption spectrum of β-carotene
|
Discussion
Accounting for the observed absorption spectrum of Chlorophyll and β-carotene
The theoretical values for the absorbance of α-chlorophyll and β-chlorophyll have approximate absorption peaks at 430 nm and 662 nm, and at 453 nm and 642 nm respectively (see Figure 1). The expected spectrum for the chlorophyll sample should have 4 distinct peaks, and the experimentally recorded sample have 3 distinct peaks that correspond with the theoretical peaks and 1 unexpected peak at 728nm. From the spectrum, the unexpected peak at 728nm is insignificant and can be attributed to poor data processing by the spectrometer. Also, despite a clear peak in the spectrograph, the data sheet failed to process a visible and significant absorbance peak at 430nm.
The experimental result for the absorption maxima of β-carotene is 448nm and falls within the expected theoretical absorption range of 440-450nm (see Figure 2). There should be another peak at approximately 470nm, but it was not reflected on the data sheet. This is due to the poor scaling of the graph which causes the overlap of the two peaks. There is an unexpected but minor absorption peak at 670nm in the experimental spectrum. This is because there is contamination of the β-carotene sample with some residual chlorophyll, which has absorption peaks at around 670nm.
Hence, the two compounds are successfully isolated.
Discussion on the procedures taken
Ethanol was used to remove water, wax or other substances that may be present on the surfaces of the leaves. These surface materials are removed as they may be insoluble in dichloromethane and therefore, prevent dichloromethane from dissolving chlorophyll and β-carotene. As chlorophyll is soluble in ethanol, the leaf is only immersed in ethanol for a short period of time. This reduces the probability of chlorophyll dissolving out and improves the accuracy of the experiment. β-carotene is insoluble in ethanol and thus, both chemicals have to be dissolved into a common solvent, ethyl acetate, to carry out the column chromatography. Every step of the experiment should be carried out in the fume hood as dichloromethane is highly volatile and an inhalation hazard. A small cotton plug is positioned at the bottom of the column to prevent alumina from being washed out of the column.
After the addition of hexane and alumina to the column, any air bubbles should be displaced. This ensures the even distribution and packing of the stationary phase so that the samples can travel evenly down the column and separation of chlorophyll and β-carotene can take place accurately.
The solvent layer should be added carefully and slowly to avoid uneven channeling. Also, the solvent layer should always completely cover the solid layer of alumina to prevent air bubbles from forming, which may lead to poor separation.
Alumina is an adsorbent stationary phase for column chromatography. It affects the flow of solvent through the column. Any polar solvents present will bind closely to alumina and flow less readily through stationary phase of the column. Thus, the polarity of the solvent directly affects the rate of elution of chlorophyll and β-carotene. Often, a series of increasing polar solvent systems are used to elute a column. Firstly, a non-polar solvent like hexane is used to elute the less polar β-carotene. Once β-carotene is eluted, a more polar solvent mixture of hexane-acetone is used to elute the more polar chlorophyll. Acetone is more polar than hexane as it has a net dipole moment.
The nature of the solvent and the order of adding the solvent must be considered. The less polar solvent, hexane, should be used first. This ensures that only the less polar β-carotene will travel rapidly down the column. Once β-carotene is eluted, a polar solvent is added to hasten the elution of the more polar chlorophyll. If the most polar solvent is used first, both chemicals will travel rapidly down the chromatography column and no separation will take place.
Also, the strength of the pump used must be moderate. If it is too fast or too slow, the isolation of chemicals will become inaccurate.
Only a slight volume of spinach pigment in dichloromethane was added to the column. This is to prevent dichloromethane, a polar solvent, from disrupting with the separation results.
A separate sample of hexane was blanked with the UV/VIS spectrometer to ensure that the absorbance of the impurities present in hexane would be factored in and eventually cancelled from the resultant spectrum of chlorophyll and β-carotene.
Accounting for why UV/VIS was absorbed
Only π –> π*, n –> π* and n –> σ* usually produce absorption in the UV/VIS region. Thus, only molecules with n or π electrons give characteristic UV/VIS spectra.
Alternating single and double bonds is called conjugation. This extended conjugated system leads to a significant π-electron delocalization. This extended π-system gains its stability by being planar as it allows all the p AOs to overlap efficiently.
This causes the energy gap between π and π* to decrease (see Figure 3) and shifts the absorbance of the molecule to longer wavelengths. It requires a photon with lesser energy and longer wavelength to excite an electron from π –> π*.
The incorporation of an atom with lone pairs of electrons can cause a shift of absorbance to longer wavelengths as n –> π* energy gap is smaller than π –> π* gap. Chlorophyll absorbs photons of longer wavelengths than β-carotene and has additional peaks at around 642 nm as it contains N and O atoms with lone pair of electrons.
Discussion on the reliability of results
Spinach pigments are organic molecules and are affected by physical conditions such as temperature, air and light. If not protected from these conditions, they can undergo hydrolysis, atmospheric oxidation and other reactions. The extracted sample of spinach pigment was heated in order to concentrate the leaf pigments and remove excess dichloromethane. This could lead to more peaks than expected. A better alternative would be to pass a gentle steam of air over the solution in a fume hood to slowly evaporate it.
Leaf pigments contain not only chlorophyll and β-carotene. It might contain other compounds such as Xanthophylls, Pheophytin A and Pheophytin B. This may result in small anomalous peaks contaminating the samples and interfere with the collected data. For example, both the absorption peaks of Xanthophylls and β-carotene are around 400 to 500 nm. Therefore, different molecules with similar conjugated systems may have similar UV/VIS spectrum.
The solvents, acetone and hexane, do not absorb UV/VIS spectrum due to the absence of a conjugated system of π bonds and atoms with lone pairs of electrons. Thus, theoretically, they should have no effect on the spectra of chlorophyll and β-carotene. However, should there be impurities in the solvents, the reliability of the results can be improved by using hexane as a blank for β-carotene and ethyl acetate as a blank for chlorophyll when doing the UV/VIS spectroscopy, instead of using hexane as a blank for both chemicals. This is because the main solvent for β-carotene is hexane and the main solvent for chlorophyll is ethyl acetate. Thus, using the respective solvents as blanks would allow for the more accurate correction of absorbance data by the spectrometer, if there are impurities in the solvent.
Instead of comparing the absorption peaks of the resultant spectra to a table of known functional groups and their associated absorbance then piecing the information before postulating about the likely structure of the molecules, the entire resultant spectra of the respective molecules was compared to their standard absorption spectra. The first approach will lead to inaccurate results as the table of absorbance values are average values and not specific to molecular species. The latter approach used in this experiment is appropriate as it will lead to more accurate results.
The cuvettes must be wiped dry and have surfaces free from fingerprints. Any surface dirt might cause the scattering of light during the UV/VIS spectroscopy. This will be misinterpreted by the spectrometer as absorbance of photons and the resultant spectra might have anomalous absorbance peaks.
The sample being used for UV/VIS spectroscopy should, ideally, be dilute and homogeneous. If the sample is concentrated, light from the light source may be scattered by the densely packed particles.Likewise, this scattering of light will be misinterpreted as absorbance by the spectrometer and may lead to inaccurate results.
Conclusion
Chlorophyll and β-carotene, having different polarities, are separated and isolated by column chromatography. Due to the conjugated system of double bonds present in both molecules, the two molecules absorb photons of longer wavelengths in the region of UV/VIS spectrum. By comparing the peak absorptions to the standard absorption peaks of the respective molecules, UV/VIS spectroscopy was used to distinguish between chlorophyll and β-carotene.The absorption peaks of chlorophyll is experimentally determined to be 456, 664 and 610 nm while that of β-carotene is 448 and 670 nm.
References
[1] http://en.wikipedia.org/wiki/File:Chlorophyll_ab_spectra2.PNG
[2] http://www.chm.bris.ac.uk/motm/carotene/absorp~3.gif
[3] http://en.wikipedia.org/wiki/Ultraviolet%E2%80%93visible_spectroscopy
[4] http://www.snypa.co.uk/OSR/UV/instrument.php
[5] http://www.scribd.com/doc/6379245/UV-SPECTROPHOTOMETER-THEORY
[6] http://www.uwgb.edu/heuerc/2D/ColorTerms.html
[7] http://141.161.23.43/S00/handout/spectrometer.htm
[8] http://www.chemlin.net/chemistry/uv_vis_spectroscopy.htm
Exercises
- Draw the structures of chlorophyll and β-carotene.
β-carotene
chlorophyll (whether it is chlorophyll A or B depends on the substituent)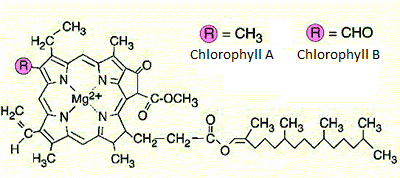
- Explain the appearance of your samples of chlorophyll and β-carotene based on your UV-visible spectrum.
β-carotene is yellow in colour while chlorophyll is green.
A conjugated system of π electrons is present in both chemicals. In addition, there are N atoms with lone pairs of electrons in chlorophyll. Such light-absorbing systems are called chromophores. The chromophores cause a major shift of absorbance to the longer wavelengths in the visible spectrum. Referring to the particle-in-a-box model, when photons with energy corresponding to the energy gap between energy levels are absorbed, its electrons are excited from a lower energy state to a higher one. The wavelengths of the photons absorbed will be displayed as absorption peaks in the spectrograph.
β-carotene absorbs photons of wavelengths 450nm. This relatively long wavelengths of light absorbed suggest that there is a small gap between energy levels and only a small amount of energy is required to excite an electron to a higher energy state, according to the equation E=hc/λ. The small energy gap is due to the extensive 11 carbon-carbon double bonds conjugation present in β-carotene.
[Figure 3] The colour that the molecule appears as would be complementary to the colour absorbed.
|
According to the UV/VIS spectra, chlorophyll has a long hydrophobic chain. It is necessary to determine the directions in which linear polarized light is preferentially absorbed in a pigment molecule. This preference corresponds to the directions of maximum electronic transition probability which in turn affects the different absorption bands of chlorophyll in the spectra obtained.
The observable colours of the two chemicals to the naked eye are complementary to the wavelengths of the colour absorbed in the visible light range of the electromagnetic spectrum (see Figure 3). Red light, with wavelengths of around 650nm, is absorbed by chlorophyll. Since the complementary colour for red is green, therefore chlorophyll will appear green in colour. Violet light, with wavelengths of around 450nm, is absorbed by β-carotene. Since the complementary colour for purple is yellow, thus β-carotene will appear to be yellow.
- For β-carotene, draw a valence-bond-molecular-orbital diagram and identify the transition during the absorption process.
There is a total of 22 adjacent p AOs as there are 22 carbon atoms that are sp2 hybridised. This will produce 11 π-bonding and 11 π-antibonding orbitals. The most stable π MO will contain no nodes and will be an MO delocalized over all 22 carbon atoms. The least stable π MO will contain 21 nodes with a node between each carbon atom in the π system.
56 σ*CH
|
MOs
|
56 σ*CH
|
MOs
| |||||
41 σ*CH
|
MOs
|
41 σ*CH
|
MOs
| |||||
π21 *MO
|
21 nodes
|
LUMO+10
|
π21 *MO
|
21 nodes
|
LUMO+10
| |||
π20 *MO
|
20 nodes
|
LUMO+9
|
π20 *MO
|
20 nodes
|
LUMO+9
| |||
π19 *MO
|
19 nodes
|
LUMO+8
|
π19 *MO
|
19 nodes
|
LUMO+8
| |||
π18 *MO
|
18 nodes
|
LUMO+7
|
π18 *MO
|
18 nodes
|
LUMO+7
| |||
π17 *MO
|
17 nodes
|
LUMO+6
|
π17 *MO
|
17 nodes
|
LUMO+6
| |||
π16 *MO
|
16 nodes
|
LUMO+5
|
π16 *MO
|
16 nodes
|
LUMO+5
| |||
π15 *MO
|
15 nodes
|
LUMO+4
|
π15 *MO
|
15 nodes
|
LUMO+4
| |||
π14 *MO
|
14 nodes
|
LUMO+3
|
π14 *MO
|
14 nodes
|
LUMO+3
| |||
π13 *MO
|
13 nodes
|
LUMO+2
|
π13 *MO
|
13 nodes
|
LUMO+2
| |||
π12 *MO
|
12 nodes
|
LUMO+1
|
π12 *MO
|
12 nodes
|
LUMO+1
| |||
π11 *MO
|
11 nodes
|
LUMO
|
π11 *MO
|
11 nodes
|
LUMO
| |||
ΔE
| ||||||||
π10 MO
|
10 nodes
|
HOMO
|
π10 MO
|
10 nodes
|
HOMO
| |||
π9 MO
|
9 nodes
|
HOMO - 1
|
π9 MO
|
9 nodes
|
HOMO - 1
| |||
π8 MO
|
8 nodes
|
HOMO - 2
|
π8 MO
|
8 nodes
|
HOMO - 2
| |||
π7 MO
|
7 nodes
|
HOMO - 3
|
π7 MO
|
7 nodes
|
HOMO - 3
| |||
π6 MO
|
6 nodes
|
HOMO - 4
|
π6 MO
|
6 nodes
|
HOMO - 4
| |||
π5 MO
|
5 nodes
|
HOMO - 5
|
π5 MO
|
5 nodes
|
HOMO - 5
| |||
π4 MO
|
4 nodes
|
HOMO - 6
|
π4 MO
|
4 nodes
|
HOMO - 6
| |||
π3 MO
|
3 nodes
|
HOMO - 7
|
π3 MO
|
3 nodes
|
HOMO - 7
| |||
π2 MO
|
2 nodes
|
HOMO - 8
|
π2 MO
|
2 nodes
|
HOMO - 8
| |||
π1 MO
|
1 nodes
|
HOMO - 9
|
π1 MO
|
1 nodes
|
HOMO - 9
| |||
π0 MO
|
0 nodes
|
HOMO - 10
|
π0 MO
|
0 nodes
|
HOMO - 10
| |||
41σCC MOs
|
82 electrons
|
41σCC MOs
|
82 electrons
| |||||
56σCH MOs
|
112 electrons
|
56σCH MOs
|
112 electrons
| |||||
40 K-shell C
|
80 electrons
|
40 K-shell C
|
80 electrons
|
According to the frontal orbital theory, an electron would be excited from the HOMO to LUMO. This electron would be one of the two electrons of π10 MO, HOMO, and it would be excited to the π11* MO, LUMO.
- Using the particle-in-a-box theory, estimate the absorption wavelength of β-carotene. Compare to the experimental value that you have obtained and comment on the difference in the wavelength.
The particle in a box can be used to roughly model the π system. The longer the conjugated system, the larger the “box” the electrons are placed in. Electrons can be localized to move only along the conjugated carbon skeleton of β-carotene. Since there are 11 carbon-carbon π bonds in the conjugated system of β-carotene, there would be 22 π electrons in the region of delocalized electrons. Using Aufbau Principle and Pauli Exclusion Principle, the electrons are paired up with opposite spin at a single energy level and occupy energy levels En from n=1 to n=11.
An electron is excited from n=11 to n=12 when the β-carotene sample undergoes UV/VIS spectroscopy. The UV/VIS absorption corresponds to an electron being removed from a π MO and being excited to a π* MO (a π –> π* transition). This makes E11 the Highest Occupied Molecular Orbital (HOMO) and E12 the Lowest Unoccupied Molecular Orbital (LUMO). Only one electron is free to move about the box and at the lowest energy state, each level has 2 electrons.
Length of C-C bond = 1.5Å
Length of C=C bond = 1.34Å
Average bond length in β-carotene = 11(1.34)+10(1.54) ≈ 30.14Å
Energy of particle in a box = n2h2/8mL2. Since the length of the box or rather, the sum of the bond lengths in the conjugate chain does not change, the equation for the energy of a particle in a box, ΔE=(n122-n112)h2/8mL2, where Planck’s constant, h=6.626x10-34Js; mass of electron, m=9.109x10-31kg, length of conjugation,L=30.14Å=30.14x10-10m.
ΔE=(n122-n112)h2/8mL2
=(122-112) (6.626x10-34) 2/8(9.109x10-31) (30.14x10-10)2
=1.525x10-19J
To calculate wavelength, use λ=hc/E, where speed of light, c=2.998x108ms-1.
λ=hc/E
=(6.626x10-34)( 2.998x108)/( 1.525x10-19)
= 1.303x10-6m
Percentage error = 190.8%
There is a large difference between the calculated value and the experimental value as seen from the significant 190.8% percentage error. This can be attributed to the fact that the particle-in-a-box theory is only a basic model of electron movement and a number of assumptions and approximations were made when the theory was applied.
The basic assumption of the model is that the electron in a box experiences zero potential energy as it moves within the length of the box and infinite potential energy outside the box. This is false as electrons in β-carotene experiences potential energy as it moves along the conjugated carbon skeleton due to electrostatic attraction to the carbon nucleus.
The second assumption is that the 22 π electrons in the conjugated system are not interacting. This is not true as there is electrostatic repulsion between the negatively charged electrons. The repulsion means that less energy is required to excite an electron from HOMO to LUMO. This in turn means that a photon of longer wavelength would be absorbed during the UV/VIS spectroscopy.
Also, there is an overestimation of the width of the box as the carbon atoms are assumed to be bonded linearly as one adds the length of the bonds numerically. This is false as the carbon skeleton chain is actually mostly trigonal planar with respect to each carbon atom. By adding the bond length linearly, there is an overestimation of the width of the box.
Due to the above reasons, the particle-in-a-box is unsuitable for the estimation of a big molecule like β-carotene as the width of the box is large. With increased width of the box, the percentage error increases. Also, there is electrostatic interaction between electron-electron and electron-nucleus; the assumption that electron has zero potential energy when in the box does not hold. Hence, the particle-in-a-box model should not for complex molecules like β-carotene and should only be used to smaller, simpler molecules.
Thank you for this comprehensive treatment of the wavelength of the beta carotene molecule.
ReplyDeleteMost textbook accounts leave one wanting more on the source of the large errors obtained when applying the particle in a box model. Other textbooks mask the error by suggesting the use of a considerably shorter 'box' length of the order of 18A as opposed to 29A for carotene.
Eric Scerri
www.ericscerri.com
You're welcome :]
DeleteHow did you find all this information? THis is the best general discussion of the sources of errors that I have found anywhere.
ReplyDeleteEric Scerri PhD
please visit my website at www.ericscerri.com
UCLA
Hi Eric, thanks for the compliment. This information has been gathered from various textbooks, websites, notes and other resources. Did it when I was an undergraduate desperately worried about my GPA.
DeleteWow, you're a very decorated chemist!
You have shared a lot of information in this article about Buchi Rotary Evaporator Parts. I would like to express my gratitude to everyone who contributed to this useful article. Keep posting.
ReplyDelete